
Mary Salazar, DNP, MSN, ANP-BC, RN; Mike Harris, MBA,
Cancer clinical trials offer patients an opportunity to be treated with the most cutting-edge and promising cancer therapies available, but the majority of patients who are offered these trials still are not signing up for them. This might frustrate those in the know, but many patients simply do not understand what these trials entail. However, navigators can play an important role in educating patients on clinical trials and making sure they do not miss out on these potentially lifesaving opportunities.
At the 2023 AONN+ Midyear Conference in Orlando, FL, Mary Salazar, DNP, MSN, ANP-BC, RN, and Mike Harris, MBA, provided some simple tools for helping patients better understand clinical trials, and, perhaps most importantly, that they will not be left with a placebo.
“They might receive the placebo instead of the study drug, but they’ll still be getting the gold standard of care, which they would have gotten had they not been involved in the trial at all,” explained Mr Harris, COO of TriCan Health, LLC, a company that matches patients with available cancer clinical trials. “I can’t tell you how many people I’ve talked to who have no idea that’s how oncology clinical trials work.”
Clinical Trials 101
Clinical trials for cancer compare the most effective known treatment for a specific type or stage of cancer with a new approach: either a new drug, a combination of drugs, or a different way of using established therapies.
“The purpose of an oncology clinical trial is to test where we are, in order to get to where we can be,” said Dr Salazar, director of Oncology Patient Experience and assistant professor in the Division of Medical Oncology at UT Health San Antonio, MD Anderson Mays Cancer Center.
Patients should understand that through these trials, healthcare providers find new ways to improve treatments and quality of life for people with cancer, as they are only comparing the most promising, state-of-the-art treatment with existing standard of care.
The first phase of a clinical trial—phase 1—evaluates safety, determines safe dosage/mode of delivery, and identifies side effects in a small group of patients (<30).
“It’s the first time this treatment is being tested in humans,” she noted. “So there’s a lot of regulation and safety surrounding the whole process.”
Phase 2 trials look at benefit vs risk by testing the effectiveness of the treatment and further evaluating its safety in 100 or fewer patients. Phase 3 trials collect more information, further confirm the effectiveness of the new treatment, and compare it with the current standard of care; these trials can involve thousands of patients, and results may or may not lead to FDA approval of the study drug. Phase 4 (postmarketing) trials are conducted after the drug’s approval and evaluate long-term side effects.
She encourages her patients to visit the National Cancer Institute website (cancer.gov), where they can access informational videos on clinical trials. Additionally, for patients interested in complementary and alternative medicines (CAMs), such as acupuncture and yoga, a long list of active CAM clinical trials is available at www.cancer.gov/about-cancer/treatment/clinical-trials/cam-procedures. “I use this a lot for my patients who are nervous about clinical trials,” she said.
Why Should Patients Participate in Clinical Trials?
Clinical trials test tomorrow’s medicine today. According to Mr Harris, they offer patients a combination of hope and access: hope that more than what they perceive is available in terms of cancer treatment, as well as access to these advanced medicines.
In addition, patients in clinical trials often receive an enhanced level of care, as the entire medical team is invested in each patient enrolled in a trial.
“The physicians involved in these trials are carefully monitoring the patients; they want to make sure there aren’t any adverse side effects, or if there are, that they’re rapidly recognized and treated,” he explained. “So oftentimes patients can actually get better care on a clinical trial than they would receiving standard of care.”
Underrepresented patients who enroll in trials can help to increase access to cutting-edge treatments for entire populations, as they will be helping to test new medicines that may be particularly effective in diverse patient populations.
Lastly, patients in clinical trials will ultimately be helping other patients with the same diagnosis.
“The concept of altruism is consistent throughout participants in clinical trials: they want to do what’s right for the whole community, including anyone else who suffers from their particular disease,” he added.
Busting Myths to Increase Patient Enrollment
According to Mr Harris, about 75% of patients who are offered cancer clinical trials express interest in learning more, but less than 8% ultimately enroll in those trials. There are a multitude of reasons for this, but many of them involve myths and widespread misinformation about clinical trials, and a common misconception that they will be treated as “guinea pigs.”
Helping patients to overcome their concerns over these myths simply involves explaining the realities (Table).
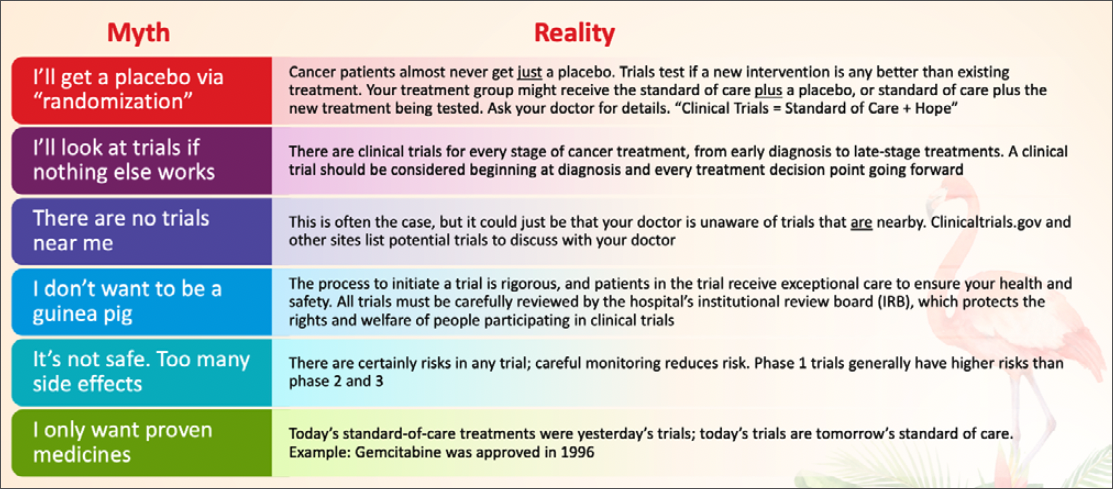
Other concerns around the trial itself might include logistics such as too much travel time, but navigators can ask about the treatment protocol for potential trials, as some require less frequent trips to the medical center. Travel resources can be found through the American Cancer Society Road to Recovery program, Medicaid Expansion Non-Emergency Transportation Programs (ie, Modivcare, MTM), and services like OncoLink, Ride Health, Envoy, GoGo, and findhelp.org.
Expense is another commonly cited barrier, but navigators should check the specifics of each trial, as trial sponsors typically pay expenses over and above the standard of care, while insurance or Medicare typically cover standard care costs.
Navigators play a crucial role in helping patients to overcome these barriers and in encouraging them to consider clinical trials throughout their entire cancer journey. To sum it up, added Mr Harris, “Clinical Trials = Standard of Care + Hope.”
More From This Expert panel
Myths and Truths About Clinical Trials






