Purpose: Women treated for breast cancer have reported lower physical and mental health status and poorer sleep quality compared with the general female population. The Breast Cancer Collaborative Registry (BCCR) from a Midwestern tertiary medical center was used to examine these factors.
Methods: This cross-sectional design used data collected from BCCR participants at the first breast cancer diagnosis. Descriptive statistics, correlations, t tests, and analysis of variance were used to analyze physical and mental health (Short- Form Health Survey [n = 548]), sleep quality (Pittsburgh Sleep Quality Index [n = 358]), and demographic/medical factors, and their relationships.
Results: The sample was predominantly Caucasian, non-Hispanic, and living with a partner; had some postsecondary education; mean age was 58.6 years; were ≤5 years postdiagnosis; had stage I or II disease; and had received cancer treatment within the past month. Physical component scores were significantly lower and mental component scores were significantly higher in this sample compared with the general female population. Sleep-quality scores were “good” (<5) in 33% but “poor” in 66% of the sample. These primary outcomes were associated with several demographic/medical factors (age, educational level, body mass index, time since diagnosis, and performance status).
Conclusions: Several explanations are provided for this sample’s reported higher mental health scores, despite self-reports of poorer physical health and sleep quality than in the general female population.
Implications for Cancer Survivors: Results confirm the need to increase physical functioning and sleep quality in breast cancer survivors. Interventions focused in these areas may need to be tailored based on mental health and other demographic/medical factors to promote adherence in survivors.
Currently, 2.9 million women in the United States are breast cancer survivors, and this number is expected to increase to 3.4 million by 2015.1,2 In this paper, survivorship is defined as beginning at diagnosis.3 Survivors seek understanding of the immediate and long-term consequences of breast cancer treatments to cure or manage the disease, including surgery, radiation, chemotherapy, and hormonal manipulation. Women who received treatment for breast cancer report consequences of lower physical and mental health and poorer sleep quality compared with the general population.4,5 Breast cancer survivors also report that decrements in physical and mental health negatively impact quality of life (QOL).6 Further understanding of the impact of breast cancer and its treatment on physical and mental functioning and sleep quality is important because of the impact on performance status and overall QOL.
A recent review of adverse physical events experienced by survivors of various types of cancer concluded that impaired physical functioning is commonly associated with fatigue and other cancer-related symptoms and often results in a decline in QOL.7-9 Persistent bodily pain that may result from cancer treatment has also been reported in breast cancer survivors. Pain can occur from multiple sources related to cancer treatment and comorbid conditions. Pain from chemotherapy-induced peripheral neuropathy is manifested as numbness, tingling, and dysesthesia, and may not resolve completely despite discontinuation of chemotherapy.10 Arthralgia and joint pain resulting from the use of aromatase inhibitors have been reported in 36% of breast cancer survivors taking these medications for prevention of recurrence.11 Upper body symptoms are reported by 10% to 64% of women following breast cancer surgery.12 Shoulder and arm tightness, pain, numbness, swelling, and limitations in mobility have adverse effects on breast cancer survivors’ physical, social, and emotional functioning.13-15
Breast cancer survivors often experience a decline in mental health in the form of prolonged stress, fear, depression, and/or anxiety. Emotional distress in many breast cancer survivors continues to be present, even long after treatment ends.16 Although depression affects 7% of the US population, the prevalence of depression is estimated to be 10% to 52% in breast cancer survivors. Elements of mental health, such as anxiety and depression, have been correlated with impaired QOL in breast cancer survivors.17,18 In a study of disease-free Finnish breast cancer survivors (n = 537), 26% of the study participants were depressed, had lower levels of QOL, and physical performance compared with the general population.19
Poor sleep quality is a prevalent symptom in women undergoing breast cancer treatment.20 Recent studies report at least 33% of breast cancer survivors have persistent sleep-wake disturbances, ranking it as one of the most common and distressing symptoms.21,22 Patients with breast cancer reported the highest number (85%) of insomnia compared with patients with other types of cancers.23 Sleep-wake disturbances include problems in sleep quality, latency, efficiency, and duration; all may impact the social, mental, and physical functioning of individuals.24 Altered patterns of sleep quantity and quality have been found to continue well into the survivorship phase.25 Increased numbers of survivors have raised awareness of the urgent need to improve the treatment of the most common cancer-related symptoms.21 Interventions aimed at reducing poor sleep in survivors have resulted in improved sleep and QOL.26-28
Local, state, and national cancer agencies, and control programs have used registry data to make public health decisions and for cancer control and epidemiologic research aimed to improve patient care outcomes.29,30 Recently, registries have included factors associated with the etiology of cancer, lifestyle, and QOL. Several cancer registries provide source material for examining factors impacting QOL in patients with breast cancer.31-33
The Pathways Study, based on the Kaiser Permanente Northern California Cancer Registry of women recently diagnosed with breast cancer (n = 950), found that younger women and women presenting with late-stage disease at diagnosis reported lower QOL; women reporting higher levels of social support had better QOL, with the exception of physical well-being.34 The study of Western Australian Cancer Registry breast cancer survivors (n = 558) who were 1 to 3 years postdiagnosis indicated that physically active breast cancer survivors within a healthy weight range reported higher QOL.35
Cancer registries can address many unanswered questions and gaps concerning breast cancer survivorship. This report addresses QOL, sleep, as well as physical and mental health in breast cancer survivors. The purpose of this study was to (1) describe the Breast Cancer Collaborative Registry (BCCR) sample with regard to physical and mental health components of QOL and sleep quality, and demographic and medical characteristics; and (2) determine the associations among physical and mental components of QOL, sleep quality, performance status, and demographic/medical characteristics of the BCCR sample.
Methods
This cross-sectional study used data from the BCCR from a Midwestern tertiary medical center.
The BCCR database is populated with 916 cases with available data from 2008 to 2011. All data were from patient interviews that were administered upon enrollment into the registry. Enrollment took place at any phase of the cancer trajectory. For this study, only women who completed the questionnaire after their initial diagnosis were considered for inclusion (n = 692). Of these, the 548 with Short-Form Health Survey (SF-36) physical and mental component summary scores formed our sample, and 358 of those had a valid total sleep quality score from the Pittsburgh Sleep Quality Index (PSQI).
This study was approved by the Institutional Review Board. Patients provided an informed consent when they enrolled in the registry for the data to be used in clinical studies. All of the participants in the BCCR were given a questionnaire at the initial study visit. This report focuses on the initial baseline data collection due to the large sample size obtained. Follow-up data are in the process of collection.
The BCCR questionnaire was designed for standard collection of data to provide a comprehensive review of factors that could influence breast cancer survivorship. The BCCR registry was developed in collaboration with consultants with expertise in breast cancer.33 The BCCR questionnaire contains extensive categories of demographic and medical data.33
Information collected included the participant’s age, race, ethnicity, marital status, height and weight, highest education level, and annual income, as well as employment status. Information was obtained to ascertain physical and mental health, sleep quality, and performance status. Other pertinent medical data were collected, including current cancer diagnosis and tumor stage, cancer treatments, and menopausal status.
Physical and mental health components were measured using the SF-36 v2 Health Survey. The SF-36 measures 8 aspects of physical and mental health limitations, including physical function, role physical, role emotional, social functioning, bodily pain, mental health, vitality, and general health. Two summary component scores were calculated, one for physical and one for mental health.30 The participants were asked to respond to the items based on the past 4 weeks (standard form). Eight subscale scores were obtained by summing items indicating the extent of limitations. Normative data are reported by gender. The SF-36 has been used in over 2000 studies, and the reliability and validity of the SF-36 are well established. Reliability scores for the 8 subscales typically exceed .80 and ranged from .80 to .95 in this sample. Version 2.0, introduced in 1996, improved the 2 role-function scales and simplified instructions and questions, thus providing greater comparability with the widely used translated and cultural adaptations.30
Sleep quality during the previous month was measured by the PSQI, a 19-item subjective tool. The total score includes 7 components: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleeping medication use, and daytime dysfunction. Components are scored on a 0 to 3 scale, then combined with equal weights to yield a global score ranging from 0 to 21. Higher scores indicate more severe complaints and poorer sleep quality. The PSQI takes less than 10 minutes to complete. Cronbach’s alpha for the global PSQI has been reported as .80 and was .72 in this study. A global PSQI score ≥5 has a sensitivity of 89.6% and a specificity of 86.5% in identifying poor sleepers. Questions 10 and 11 (optional) that were to be filled out by a bed partner or roommate were not included.29
Performance status was measured using an adaptation of the Eastern Cooperative Oncology Group instrument to assess how the cancer experience affects daily activities.36 One item in the BCCR questionnaire asked participants to rate their level of physical activity over the past week. Response options were ranked from 1 to 5 and were coded as follows: (1) fully active (able to perform all activities without restriction); (2) restricted in strenuous activity (can walk, able to carry out light housework); (3) can walk and take care of self (up more than 1/2 day); (4) need some help taking care of self (spend more than 1/2 day in bed or chair); and (5) cannot take care of self at all (spend all of the time in bed/chair). Based on the authors’ data, because less than 5% of the sample reported restrictions greater than option 2, categories 2 through 5 were merged and the category was labeled as “some restrictions.”
The data were entered and verified by 3 research assistants who received training in the BCCR database. Descriptive statistics (frequency distributions or means and standard deviations) were obtained for demographic and medical variables, physical and mental health, sleep quality, and performance status. Sample means for the SF-36 subscales and component scores were compared with women in the population norming sample using a 2-sided single-sample z test.37 Correlations were calculated for the SF-36 physical health component scores, mental health component scores, and PSQI total scores. For categorical background variables, t tests or one-way analysis of variance (ANOVA) were conducted to compare category means on sleep and the SF-36 component scores. All tests was conducted using α = .05, unless otherwise noted.
Results
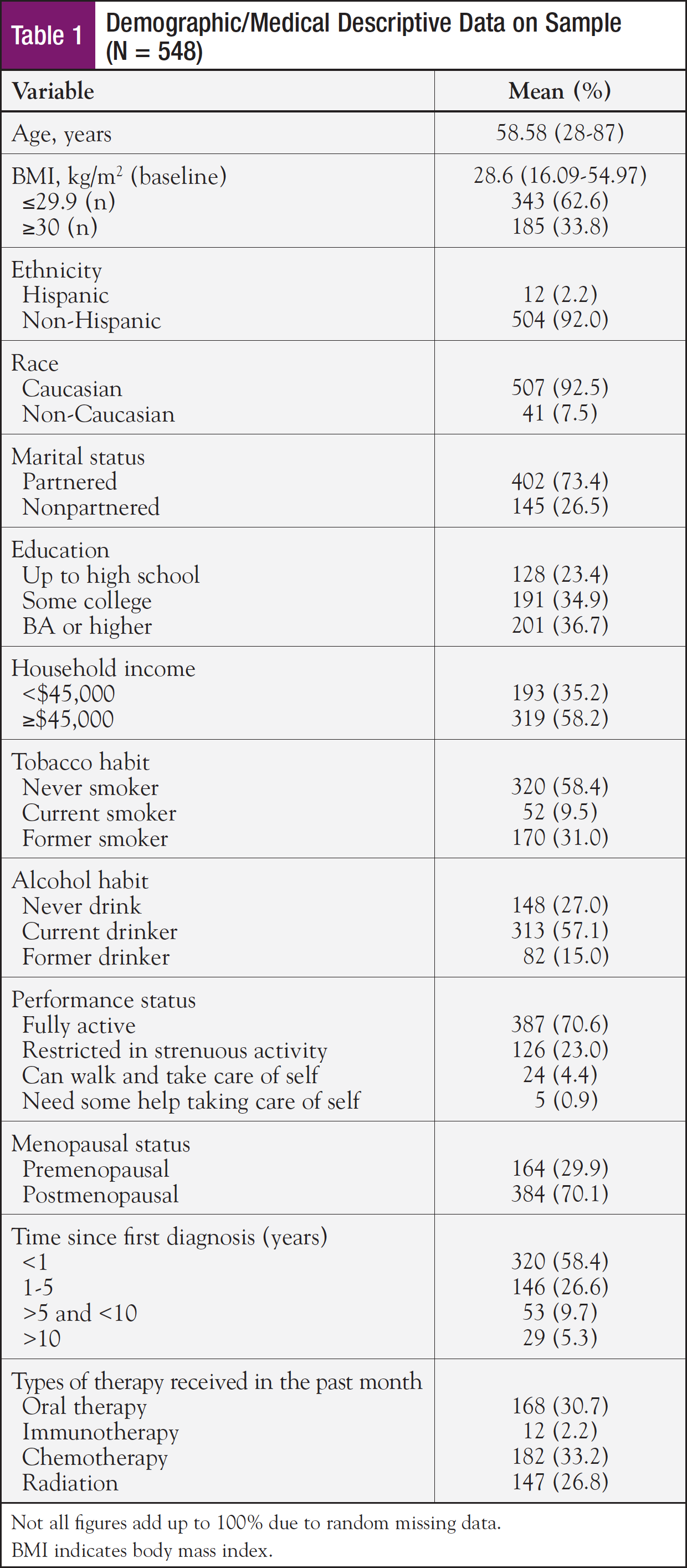
Demographic and medical characteristics of the sample are displayed in Table 1. The sample was predominantly Caucasian, non-Hispanic, living with a partner, with at least some postsecondary education, and an annual household income at or exceeding $45,000. The mean age was 58.6 years, and approximately two-thirds of the sample self-reported being postmenopausal. Three-fourths of the sample were within 5 years of a breast cancer diagnosis. Pathology reports indicated the majority of the sample had stage I or stage II tumors. Most of the sample had received at least 1 form of cancer treatment within the past month; chemotherapy and oral therapy were the most common treatments.
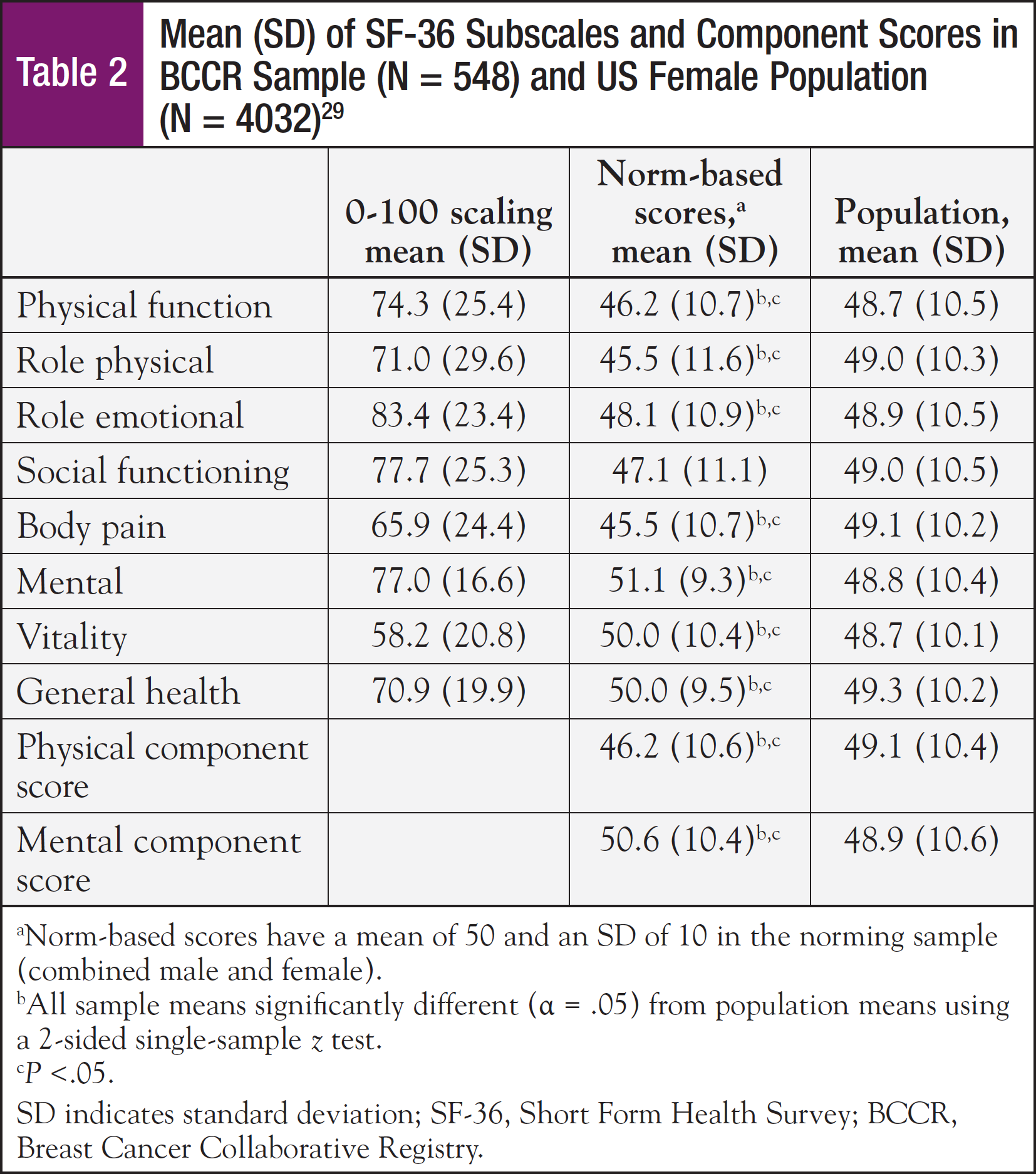
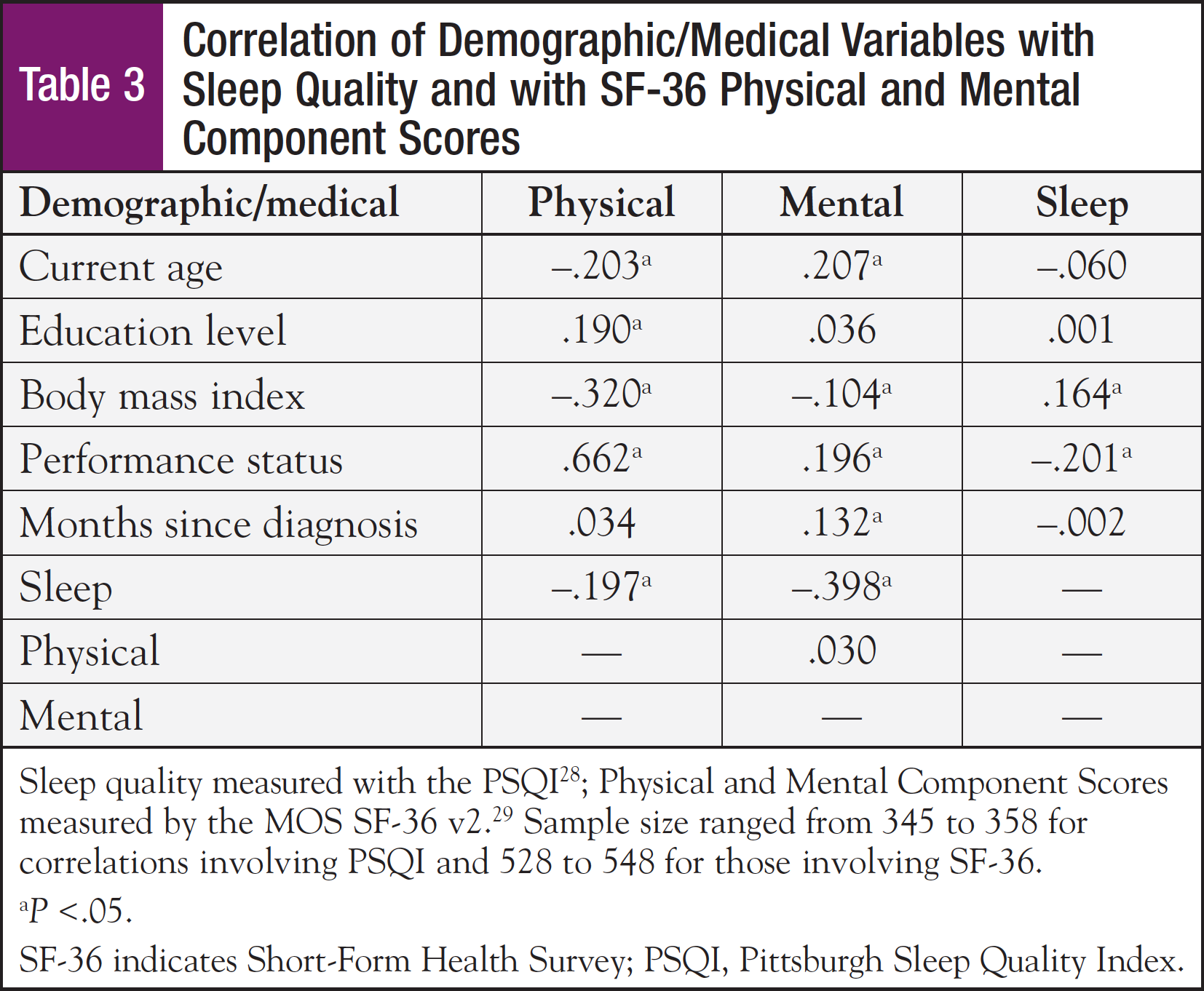
Mean SF-36 subscale scores for the physical functioning, role physical, and bodily pain subscales, and also the mean physical component summary score were significantly lower for this sample compared with the general population (Table 2). However, this sample rated their general health status higher than the general population. Older age, lower education level, higher body mass index (BMI), having some restrictions in performance status, and poor sleep quality were all significantly associated with lower physical component summary scores (Table 3). Approximately 70% of the sample reported their performance status as being fully active, performing usual activities without restriction. The remaining 30% required assistance, with some restrictions in performance. Performance status was associated with both physical and mental component scores and sleep quality.
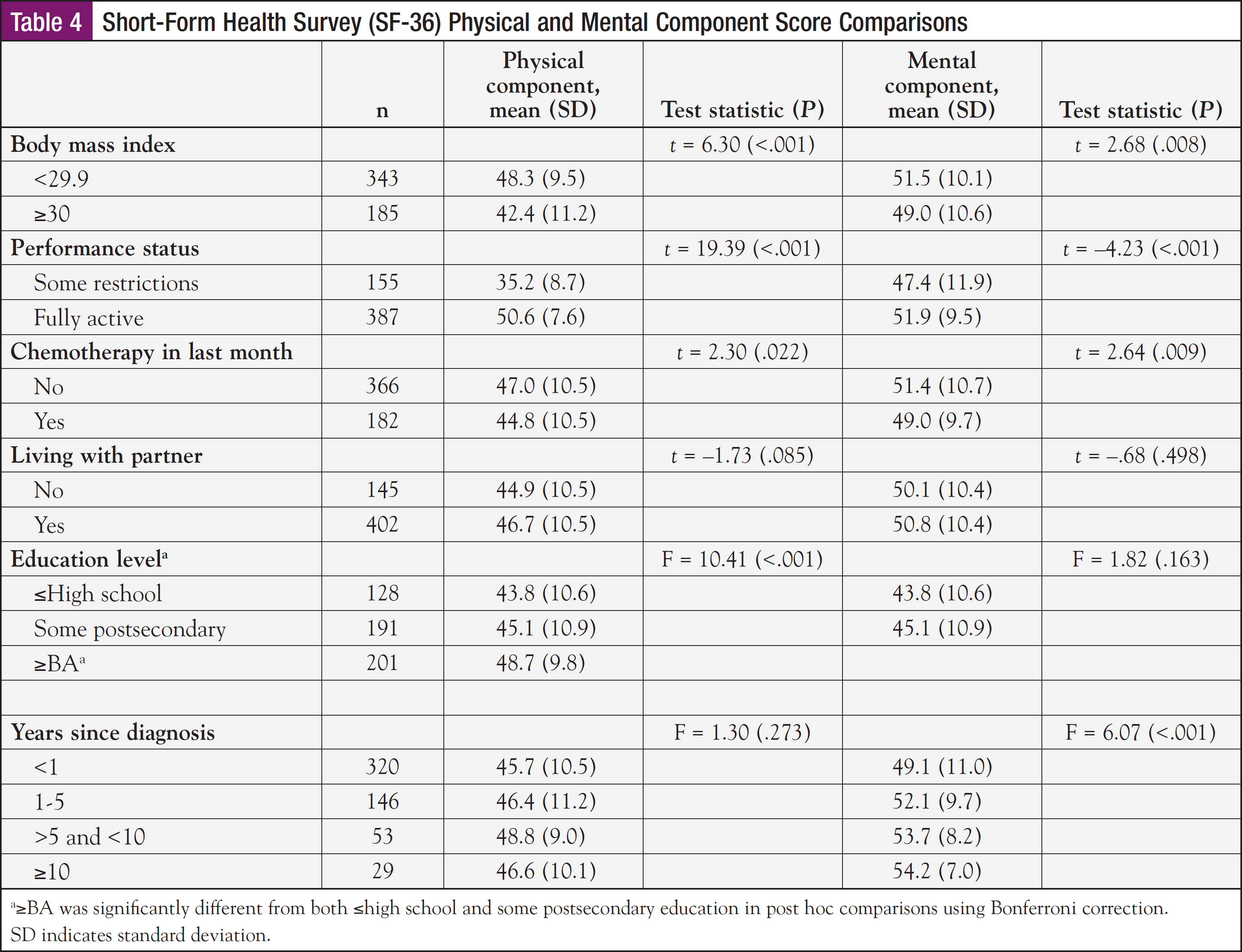
With respect to mean SF-36 mental subscale scores, the sample means for the role emotional and social functioning subscales were significantly lower, while the mental and vitality subscales were higher than in the general female population (Table 2). Younger age, higher BMI, fewer months since diagnosis, having some restrictions in performance status, and poor sleep quality were significantly associated with lower mental component scores (Table 3). The t tests revealed that higher BMI, some restrictions in performance status, chemotherapy treatment within the past month, and <1 year from diagnosis were factors that were significantly associated with lower mental health component scores (Table 4).
Of the 358 patients with a PSQI total score, approximately 33% reported good sleep quality (<5). However, 66% reported poor sleep quality (≥5), and in more than half of those cases, the PSQI score was >8, a cutoff score reflective of poor sleep in patients with cancer.38 Poorer sleep quality was associated with a higher BMI, some restrictions in performance status, and lower physical and mental component scores. The t tests and ANOVA revealed that higher BMI (P = .002), some restrictions in performance status (P = .001), and some postsecondary education (P = .021) were significantly associated with poorer sleep quality. ANOVA revealed perceived sleep quality varied by education level. Post hoc comparisons showed that survivors with some postsecondary education reported poorer sleep than those with a high school education or less (P = .015).
Discussion
This cancer registry study examined the physical and mental health components of QOL, sleep quality, and demographic and medical characteristics of breast cancer survivors, and the associations among these variables. A major finding consistent with previous reports in the literature was that these breast cancer survivors reported lower overall physical functioning. Although the majority of the survivors rated themselves as fully physically active on a gross measure of performance status, a more sensitive measure indicated lower physical functioning than the general population. Sleep quality was poor for the majority of survivors in this sample, a finding consistent in patients with breast cancer, especially while they are actively receiving treatment. A major finding that was not consistent with previous studies was that this sample of survivors reported higher mental health scores than in the general female population. These findings will be discussed, strengths and weaknesses identified, and implications proposed.
Physical Health
Based on the authors’ observations, lower physical functioning was prevalent in this sample, most of whom were in the first year following diagnosis. The lower physical component score is congruent with published literature.The lower scores for several physical subscales are consistent with other studies of cancer survivors.39,40 This registry study confirmed previous findings that the impact on physical functioning is more severe in women undergoing chemotherapy4 compared with women receiving other cancer treatments.9 Other studies consistently report significant declines in physical functioning in the first 5 years following a breast cancer diagnosis, which dissipate with further elapsed time.31,41 However, physical functioning in this sample was not higher in survivors who were ≥10 years postdiagnosis, perhaps due to comorbid diseases and aging. Though differences were not significant, the pattern of means is consistent with descriptive study reports of higher physical functioning in the 5- to 10-year posttreatment phase.42,43
The present study agrees with previous reports of bodily pain in women who received breast cancer treatment.10-15 However, this study was not able to determine the exact source of respondents’ pain. Breast cancer survivors who are overweight or obese have reported higher pain levels compared with normal weight survivors.42,43 One-third of the BCCR participants were classified as obese, and obesity may be one potential contributor to the bodily pain reported by this sample. This study also supports a relationship between higher education level and higher physical functioning in the general population.9
Mental Health
Although some of the SF-36 subscale scores were lower in this sample than in the general population, the mental component scores were higher. This study’s findings contradict previous reports of lower mental health scores in breast cancer survivors, frequently reported as emotional distress, anxiety, and depression.16,17,25 Several studies found the SF-36 mental component scores of breast cancer survivors to be higher after chemotherapy ended.44,45,46 However, a study from France reported no association between treatment type and mental component scores in long-term breast cancer survivors.47
These findings may be explained by the demographic characteristics of the BCCR sample. The sample was drawn from an urban cancer center in the Midwest that patients from surrounding rural communities, a demographic area with reported higher mental health scores in the general population. Other factors such as the relatively higher age (mean, 58.5 years), education level, household income, partnered status, and performance status may also have affected this result and may account for the higher mental health scores reported in this sample.
Several demographic variables were associated with poorer mental health in this sample. Higher BMI has been associated with lower mental health scores after the chemotherapy treatments end.48 Consistent with the literature, survivors with less time since diagnosis had poorer mental health, with this relationship reported as persisting 5 to 10 years postdiagnosis.31
The literature is inconsistent regarding the association between age and mental health in breast cancer survivors. In Latina survivors, older age has been associated with poorer mental health.48 This study found poorer mental health in younger, primarily Caucasian women. Younger age has been associated with increased depressive symptoms49 and a more negative impact on psychosocial functioning.49,50 This finding may be related to differences in partner relationships, body image, sexual functioning, and coping mechanisms in younger women with breast cancer.46 Previous research supports feelings of negative family impact, career disruption, and anxiety due to the potentially more severe illness that is often present in younger women with breast cancer.51,52
Sleep Quality
Most women have reported a high number of night time awakenings during and after breast cancer treatment.53 Studies have confirmed that changes in sustained sleep after breast cancer diagnosis are related to the degree and duration of other cancer-related symptoms such as fatigue, distress, depression, low physical activity levels, and lower QOL.25,51,54 The findings from this study further support that poor sleep quality is associated with poorer physical and mental health. The associations between poor sleep quality, higher BMI, and lower performance status were consistent with current literature.55 Results regarding poorer sleep after breast cancer diagnosis in women with some postsecondary education contradict literature associating higher risk of poor sleep in women with less education. The factors that may underlie poor sleep in this sample cannot be determined by the registry data. However, early identification of poor sleep quality among women with breast cancer who have a higher BMI, restricted physical performance, and poor mental health can assist the nurse navigator in preventing further decrements in physical and mental health that can persist well into survivorship.
Breast Cancer Registry
Cancer registries can be useful in uncovering short- and long-term issues related to breast cancer survivorship, specifically for physical and mental health and overall QOL. At present, few studies use population-based cancer registries for advancing knowledge of breast cancer survivorship. The majority of registry studies have used a cross-sectional design and thus lack generalizability. Findings from the BCCR support the findings of 2 other population-based cancer registries that suggest physical functioning impairments occur with breast cancer treatments and these impairments are influenced by aging.31,48 However, 1 registry study52 found younger women had better well-being but worse overall QOL. These participants were recruited and measures taken in the immediate postdiagnosis period. In contrast, the BCCR study enrolled participants in all phases of the cancer survivorship trajectory. In another registry study, physically active breast cancer survivors with a normal BMI reported higher QOL.35
Strengths of this study include the large sample and inclusion of a measurement of sleep quality. The large number of breast cancer registry participants provided additional insights into survivorship issues associated with recovery from breast cancer treatment. Sleep quality can affect QOL, but its measurement is generally not included in cancer registries. Several limitations to this report have been identified. These include the cross-sectional design and a high percentage of early-phase breast cancer survivors. The measure of performance status was a single-item question that addressed “activity levels” only and thus provided limited data. The SF-36 measurement did not permit us to determine the reason for bodily pain present in this sample of breast cancer survivors.
Population-based cancer registries provide an opportunity for nurse researchers to examine important clinical, demographic, and individual variables in order to further understand how these factors impact breast cancer survivorship. Implications for future research include understanding the individual factors that may negatively impact survivorship and recognizing the need to develop interventions to improve physical function in all survivors, and specifically for those who enter treatment with a higher BMI, poor sleep, and lower performance status. Implications for practice for the nurse navigator include assessment and monitoring of physical and mental health, sleep disturbances, and other symptoms from the time of diagnosis, during treatment, and throughout survivorship. Nurse navigators are in a pivotal position to address individual patient factors that may impact QOL in breast cancer survivors. Patients with persistent pain, depression, anxiety, younger age, higher BMI, and poor sleep who are newly diagnosed and have some restrictions in performance status are at risk for poor QOL. Nurse navigators should recommend that clinical care for sleep disturbances and physical and mental health issues be provided promptly to enhance QOL among women with breast cancer. Furthermore, breast cancer survivorship programs should be initiated early and tailored to the breast cancer survivor’s individual needs. It is particularly important to address the needs of patients most at risk for poor sleep quality, which can lead to decrements in physical and mental health and impaired overall QOL.
Acknowledgments: Kenneth Cowan, MD, PhD; Simon Sherman, PhD; and Elizabeth Fleissner, BS, RN.
Author Disclosure Statement: All authors have nothing to disclose.
Corresponding Author: Constance Visovsky, PhD, RN, ACNP-BC, University of South Florida, 12901 Bruce B. Downs Blvd, MDC 22, Tampa, FL 33612-4766. E-mail: cvisovsk @health.usf.edu.
References
- American Cancer Society. Cancer Facts & Figures 2013. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013. Accessed May 5, 2014.
- Ellsworth RE, Valente AL, Shriver CD, Bittman B, Ellsworth DL. Impact of lifestyle factors on prognosis among breast cancer survivors in the USA. Expert Rev Pharmacoecon Outcomes Res. 2012;12(4):451-464. doi:10.1586/erp.12.37.
- Nevidjon B. Evolution of survivorship care. In: Lester JL, Schmitt P, eds. Cancer Rehabilitation and Survivorship: Transdisciplinary Approaches to Personalized Care. Pittsburgh, PA: Oncology Nursing Society; 2011:1-2.
- Berger AM, Lockhart K, Agrawal S. Variability of patterns of fatigue and quality of life over time based on different breast cancer adjuvant chemotherapy regimens. Oncol Nurs Forum. 2009;36(5):563-570.
- Huang SM, Tai CJ, Lin KC, Tai CJ, Tseng LM, Chien LY. A comparative study of symptoms and quality of life among patients with breast cancer receiving target, chemotherapy, or combined therapy. Cancer Nurs. 2013;36(4): 317-325.
- Smith AW, Alfano CM, Reeve BB, et al. Race/ethnicity, physical activity, and quality of life in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2009;18(2):656-663.
- Brearley SG, Stamataki Z, Addington-Hall J, et al. The physical and practical problems experienced by cancer survivors: a rapid review and synthesis of the literature. Eur J Oncol Nurse. 2011;15(3):204-212.
- Cheville AL, Troxel AB, Basford JR, Kornblith AB. Prevalence and treatment patterns of physical impairments in patients with metastatic breast cancer. J Clin Oncol. 2008;26(16):2621-2629.
- Sehl ME, Satariano WA, Ragland DR, Reuben DB, Naeim A. Attribution of functional limitation to cancer decreases in the year following breast cancer diagnosis in older patients. Crit Rev Oncol Hematology. 2009;71(1):62-69.
- Stubblefield MD, McNeely ML, Alfano CM, Mayer DK. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012;118(suppl 8): 2250-2260.
- Niravath P. Aromatase inhibitor-induced arthralgia: a review. Ann Oncol. 2013;24(6):1443-1449. doi:10.1093/annonc/mdt037. Epub 2013 Mar 6.
- Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118 (suppl 8):2237-2249.
- Hayes SC, Rye S, Battistutta D, DiSipio T, Newman B. Upper-body morbidity following breast cancer treatment is common, may persist longer-term and adversely influences quality of life. Health Qual Life Outcomes. 2010;8:92.
- Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
- Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol. 2002;20(20):4242-4248.
- Deshields T, Tibbs T, Fan MY, Taylor M. Differences in patterns of depression after treatment for breast cancer. Psychooncology. 2006;15(5):398-406.
- Zainal N Z, Nik-Jaafar N R, Baharudin A, Sabki ZA, Ng C. Prevalence of depression in breast cancer survivors: a systematic review of observational studies. Asian Pac J Cancer Prev. 2013;14(4):2649-2656. doi:http://dx.doi.org/10.7314/APJCP.2013.14.4.2649.
- Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8/CD007566.
- Penttinen H M, Saarto T, Kellokumpu-Lehtinen P, et al. Quality of life and physical performance and activity of breast cancer patients after adjuvant treatments. Psychooncology. 2011;20(11):1211-1220. doi:10.1002/pon.1837. Epub 2010 Sep 27.
- Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs. 2010;14(2):101-110.
- Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26(5):768-777.
- Roscoe JA, Kaufman ME, Matteson-Rusby SE, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(suppl 1):35-42.
- Palesh OG, Roscoe JA, Mustian KM, at al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center—Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292-298.
- Alfano CM, Lichstein KL, Vander Wal GS, et al. Sleep duration change across breast cancer survivorship: associations with symptoms and health-related quality of life. Breast Cancer Res Treat. 2011;130(1):243-254.
- Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage. 2002;24 (5):471-480.
- Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer [published correction appears in J Clin Oncol. 2010;28(19):3205]. J Clin Oncol. 2008;26(28):4651-4658.
- Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23(25):6083-6096.
- Langford DJ, Lee K, Miaskowski C. Sleep disturbance interventions in oncology patients and family caregivers: a comprehensive review and meta- analysis. Sleep Med Rev. 2012;16(5):397-414.
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213.
- Ware JE Jr. SF-36 health survey update. Spine. 2000;25(24):3130-3139.
- Klein D, Mercier M, Abeilard E, et al. Long-term quality of life after breast cancer: a French registry-based controlled study. Breast Cancer Res Treat. 2011;129(1):125-134.
- Martino M, Ballestrero A, Zambelli A, et al. Long-term survival in patients with metastatic breast cancer receiving intensified chemotherapy and stem cell rescue: data from the Italian registry. Bone Marrow Transplant. 2013; 48(3):414-418.
- Sherman S, Shats O, Fleissner E, et al. Multicenter breast cancer collaborative registry. Cancer Inform. 2011;10:217-226.
- Kwan ML, Ergas IJ, Somkin CP, et al. Quality of life among women recently diagnosed with invasive breast cancer: the Pathways Study. Breast Cancer Res Treat. 2010;123(2):507-524.
- Milne HM, Gordon S, Guilfoyle A, Wallman KE, Courneya KS. Association between physical activity and quality of life among Western Australian breast cancer survivors. Psychooncology. 2007;16(12):1059-1068.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6): 649-655.
- Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF-36 Health Survey. Lincoln, RI: QualityMetric Inc; 2000.
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45(1):5-13.
- Zhao G, Li C, Li J, Balluz LS. Physical activity, psychological distress, and receipt of mental healthcare services among cancer survivors. J Cancer Surviv. 2013;7(1):131-139.
- Maly RC, Stein JA, Umezawa Y, Leake B, Anglin MD. Racial/ethnic differences in breast cancer outcomes among older patients: effects of physician communication and patient empowerment. Health Psychol. 2008;27(6): 728-736. doi:10.1037/0278-6133.27.6.728.
- Casso D, Buist DS, Taplin S. Quality of life of 5-10 year breast cancer survivors diagnosed between age 40 and 49. Health Qual Life Outcomes. 2004;2:25.
- Ganz PA. Quality of life assessment in breast cancer: when does it add prognostic value for survival? [editorial] Breast J. 2011;17(6):569-570.
- Keating NL, Nørredam M, Landrum MB, Huskamp HA, Meara E. Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc. 2005;53(12):2145-2152.
- Forsythe LP, Alfano CM, George SM, et al. Pain in long-term breast cancer survivors: the role of mass index, physical activity, and sedentary behavior. Breast Cancer Res Treat. 2013;137(2):617-630.
- Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29(9):1101-1109. doi:10.1200/JCO.2010.28.8043. Epub 2011 Feb 7.
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386-405. doi: 10.1093/jnci/djr541. Epub 2012 Jan 23.
- Tessier P, Lelorain S, Bonnaud-Antignac A. A comparison of the clinical determinants of health-related quality of life and subjective well-being in long-term breast cancer survivors. Eur J Cancer Care (Engl). 2012;21(5):692-700.
- Azuero A, Benz R, McNees P, Meneses K. Co-morbidity and predictors of health status in older rural breast cancer survivors. Springerplus. 2014;3:102. doi:10.1186/2193-1801-3-102. eCollection 2014.
- Broeckel JA, Jacobsen PB, Balducci L, Horton J, Lyman GH. Quality of life after adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2000;62(2):141-150.
- Avis NE, Crawford S, Manuel J. Psychosocial problems among younger women with breast cancer. Psychooncology. 2004;13(5):295-308.
- Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: quality of life of young breast cancer survivors. Psychooncology. 2004;13(3):147-160.
- Costanzo ES, Lutgendorf SK, Mattes ML, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br J Cancer. 2007;97(12):1625-1631.
- Dhruva A, Paul SM, Cooper BA, et al. A longitudinal study of measures of objective and subjective sleep disturbance in patients with breast cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2012; 44(2):215-228.
- Otte JL, Carpenter JS, Russell KM, Bigatti S, Champion VL. Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors. J Pain Symptom Manage. 2010;39(3):535-547.
- Payne J, Piper B, Rabinowitz I, Zimmerman B. Biomarkers, fatigue, sleep, and depressive symptoms in women with breast cancer: a pilot study. Oncol Nurs Forum. 2006;33(4):775-783.