Mike Harris, MBA
Cofounder, Trican Health
Jennifer West, BSN, RN, CCRC, ONN-CG
University of Florida
Mary Salazar, DNP, MSN, ANP-BC, RN
Director of Oncology Patient Experience, UT Health San Antonio



Mike Harris, MBA; Jennifer West, BSN, RN, CCRC, ONN-CG; Mary Salazar, DNP, MSN, ANP-BC, RN
Executive Summary: Clinical trials for cancer treatments offer patients invaluable access to emerging therapies but are not always well understood by patients or even the medical community. Closing the gap between high patient interest levels and low patient enrollment will help thousands of patients benefit from cutting-edge therapies while accelerating advances for future patients.
Clinical Trials Defined
The American Cancer Society defines clinical trials as “research studies that use human volunteers to test new drugs or other treatments to compare current, standard treatments with others that may be better. They may also test new ways to diagnose or prevent a disease.”1
Research scientists and oncologists continuously work to improve health outcomes for cancer patients. When developing new drugs, procedures, and devices to treat people with cancer, a critical part of the development process is to test these new treatments with human volunteers over various phases of increasingly large trials. Each phase of the trial is a sort of test to make sure that the trial should advance to the next phase. Volunteers are carefully screened against inclusion and exclusion criteria specific to each trial and are then carefully monitored throughout treatment to test for both safety and efficacy.
Nearly every treatment used today was previously evaluated in a clinical trial.2 In that sense, yesterday’s trials enabled today’s treatments, and today’s trials will enable the treatments of tomorrow. Figure 1 shows a simplified graphic describing the main phases of a clinical trial. The National Institutes of Health has an excellent resource page that offers more details.3
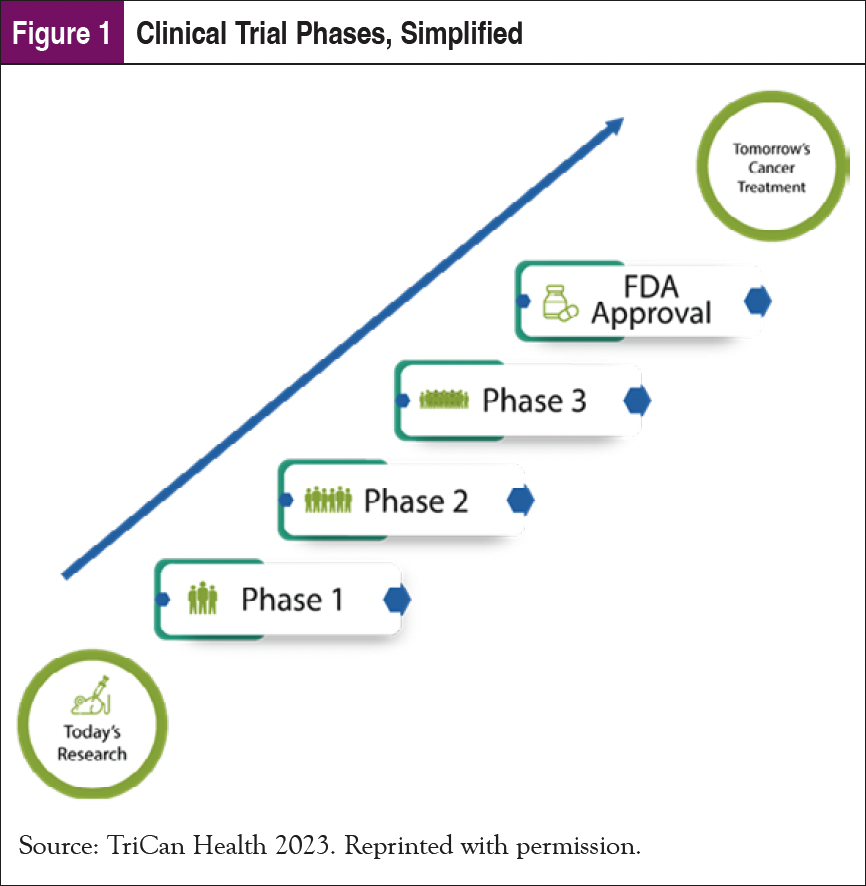
The criticality of clinical trials in the advancement of cancer treatment is at odds with the very small proportion of patients who ultimately enroll in clinical trials. That chronic problem is even more severe for underrepresented populations.
Importance of Diversity
An FDA Center for Drug Evaluation and Research study comprised of 32,000 US patients participating in drug trials in 2020 highlights the extent of the challenge with diversity in clinical trials.4 Of those patients participating in clinical trials, 75% were non-Hispanic White, while that same demographic group comprises 59% of the US population.5 Hispanics comprised 11% of trial participants, far below the 19% of the US population they represent. And Blacks or African Americans made up 8% of trial participants vs 14% of the US population. That is a problem since there can be clinical response variations by different ethnicities. Without accurately representing the patient population in clinical trials, researchers might not fully understand how new medicines work in different populations.
Oncology care and clinical trials have had diversity gaps for many years, and the pandemic proved that those gaps persist. Key thought leaders across the country have looked to oncology navigators to help close the diversity gap in clinical trials long before the pandemic.6 Those efforts will receive a boost from the recent Diverse and Equitable Participation in Clinical Trials Act, part of the 2023 omnibus spending bill, which requires diversity action plans for the clinical trials used by the FDA to decide whether drugs are safe and effective.7
Geographic Diversity
The need for diversity in trial participation extends beyond racial and ethnic diversity. It also applies to rural and urban trial enrollment. Although 74% of cancer patients are treated in community hospitals, the vast majority of trials are run in urban academic medical centers. A 2021 study published in the Annals of Surgical Oncology notes that “Age, race, insurance, and geography are barriers to clinical trial enrollment,” noting that living in the American south is a statistically significant factor in decreased clinical trial participation.8
The Gap Between Interest and Enrollment
Clinical trial enrollment overall, in every region and across all ethnicities, is well below where it could or should be. The Perceptions and Insights Study 2021 by the Center for Information and Study on Clinical Research Participation highlights that roughly 77% of patients are willing to participate in a clinical trial (30% are Very Willing, 47% are Somewhat Willing).9 But in the United States, only 2% to 8% of eligible cancer patients ultimately enroll in a clinical trial.10
So, what are the reasons for such low clinical trial awareness and enrollment, considering that when patients are made aware of trial options, a large proportion agree to participate? An important contributor is the prevalence of myths that persist among patients and even in the medical community. This article seeks to dispel some of the most common myths about clinical trials in oncology by providing their associated truths.
Myths and Truths of Clinical Trials
Myth #1: Placebos
Many cancer patients avoid clinical trials because they believe they might receive nothing more than a sugar pill as a placebo instead of receiving experimental treatment or the standard of care.
Truth
The purpose of a clinical trial is to compare the safety and efficacy of a new therapy vs the existing standard of care. Some trials use placebos to prevent bias within the study. Patients will be made aware if their study includes placebos, but they won’t know if they personally are receiving a placebo or not. Importantly, if there is a treatment available for their cancer, they will receive treatment. If a placebo is used in an oncology clinical trial, it’s typically used in addition to the standard of care, not in place of standard of care.11
Myth #2: Randomized
This myth is closely associated with the placebo myth and often arises when a potential trial participant learns that assignment into treatment groups is randomized (and potentially blinded as well). The patient’s concern is understandable—they won’t know or have control over their placement in the trial.
Truth
An important element to communicate to patients about randomization in cancer clinical trials is that at the time of enrollment it is not known which treatment group is best for the patient or to which arm they will be assigned.12 This is important because typically the control group receives standard therapy, while the investigational group receives the new therapy. Patients may not understand that they will be receiving care for their cancer in a randomized trial; it just might be the standard care that most patients receive for that form of cancer (Figure 2).
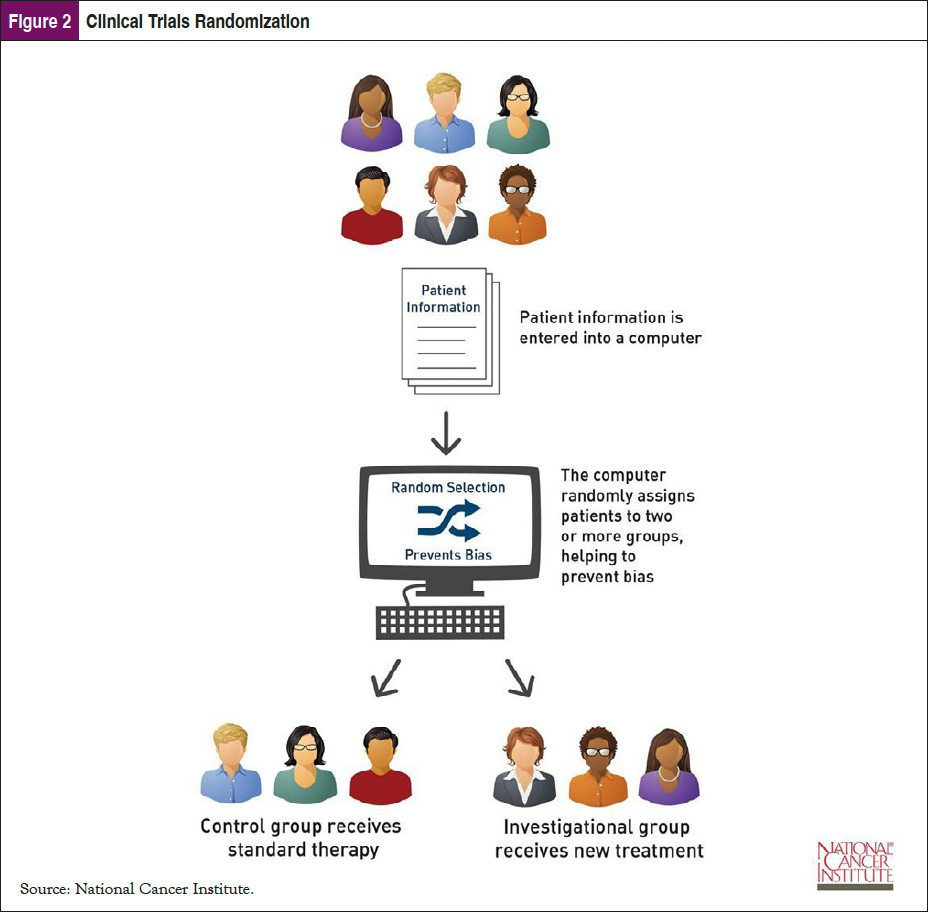
Myth #3: Guinea Pigs
Some patients have expressed that they do not want to be “guinea pigs” used to test unproven and potentially unsafe medicines without any benefit to themselves.
Truth
Most patients (and some medical professionals) are unaware of the rigorous testing and the FDA requirement for an Investigational New Drug application process for a new treatment to even make it to a clinical trial.13 It takes years of research and many approval steps before any drug makes it to a clinical trial. All patients who enroll in trials receive exceptional attention and care from the medical team. The new treatment only makes it to the clinical trial stage because research indicates it has the potential to deliver better patient outcomes—longer progression-free survival, fewer side effects, or both. Unlike guinea pigs, clinical trial participants may directly benefit from the treatment being tested.
Myth #4: Trials Should Be Considered When All Other Treatments Have Failed
Many patients and some healthcare providers think that clinical trials are only available when the standard of care has failed.
Truth
This could not be further from the truth. Clinical trials are open to all cancer patients at all stages of their diagnosis and should be part of the discussion from the time of initial diagnosis. The National Comprehensive Cancer Network provides guidelines representing the collective wisdom of how cancer should be treated, and they very expressly advocate clinical trial participation: “The best management for any patient with cancer is within a clinical trial. Clinical trials offer patients access to the most current cancer care, treatment by experts, carefully monitored results tracking, and the ability to help other patients with cancer.”14
With evolving genetic and molecular testing and the expansion of immunotherapies and targeted therapies, new treatments are constantly being tested in clinical trials, opening many new options for patients to consider. Patients and healthcare providers should be educated on the role of trials and the accessibility of these options for their patients.
Myth #5: Clinical Trials Are Too Expensive/Not Covered by Insurance
Many patients think that participating in a clinical trial is too expensive or may not be covered by their insurance.
Truth
Most health insurance policies are required by law to cover the routine costs of a clinical trial.15 This would include care that is normally done as part of standard of care. They cannot deny insurance coverage as long as the trial is an approved trial and the patient is not seeking care outside of their network. The insurance company does not have to pay for trial-related visits, the study drug, or additional blood work or scans that are specifically related to the clinical trial itself. Those trial-related visits and treatments are often covered by the trial sponsor. Patients who are insured through the Veterans Administration can participate in a clinical trial and could potentially be reimbursed for costs if the trial is a National Cancer Institute–designated trial.
Navigators can help patients understand their financial responsibility by having a basic knowledge and understanding of clinical trials and the resources that are available.
Myth #6: Logistical Challenges Make Clinical Trials Impossible
Patients can be overwhelmed by logistical challenges when considering clinical trials, particularly when the trial site is far away from the patient’s home/work.
Truth
Facility access can definitely pose logistical challenges for patients hoping to participate in a clinical trial. Depending on the trial design, a patient may be able to participate in a trial but still receive their care locally. Alternate approaches can use telemedicine visits to follow-up on drug compliance and side effect reporting. Lab draws can sometimes be done at local facilities, and drugs can be shipped directly to the patient. Additionally, many trials are designed to coincide with standard-of-care visits, making it easier for patients to participate. Some sponsors are also willing to offset the cost of necessary travel via assistance with lodging or by providing a travel stipend.16
Efforts to Close the Gap
Increasing patient enrollment in cancer clinical trials is a worthy goal for the medical community. Doing so will provide access to advanced medicines to a broader cohort of patients, particularly in those populations currently underrepresented. It has the opportunity to increase the quality and duration of life for cancer patients willing and able to explore their treatment options. And while clinical trials have many challenges and myths to overcome, there are some important industry trends and valuable resources that are helping to do so.
Matching Patients to Clinical Trials
To date, the process of matching a patient to an actively recruiting clinical trial has been challenging, in part due to the complexity of inclusion and exclusion criteria, and in part due to dissimilarities in patient health records and trial enrollment requirement descriptions.
Fortunately, robust clinical trials information can be found on websites run by the US government, domestic and international foundations, alliances, and professional organizations. The site www.clinicaltrials.gov lists active and completed trials across the United States and around the world. Other foundations and alliances that have traditionally been disease-site specific, such as the Pancreatic Cancer Action Network (https://pancan.org/), the Colorectal Cancer Alliance (www.ccalliance.org/), and LUNGevity (www.lungevity.org/), also list trials and provide research initiatives and navigation services to directly assist patients in finding trials.
Artificial intelligence (AI) has proved to be very helpful in this space. According to Beck and colleagues in their 2020 study utilizing AI to help screen patients, agreement was found to be 81% to 96% when comparing system and manual assessment of eligibility determinations.17 Utilizing AI to assist with matching patients to trials is known as “clinical trial matching” and has proved to demonstrate its worth in quickly getting patients to trials that match their precise tumor biology. Another study by Haddad and colleagues demonstrated accuracy, sensitivity, and specificity of >80% when using AI to determine eligibility of patients for clinical trials.18
Decentralization of Clinical Trials
Some of the biggest problems with enrollment in clinical trials are the logistical and financial barriers that make it difficult for patients to participate. Many of the clinical trials that are enrolling are being conducted at large academic facilities, often in city centers. That in itself can be a big hurdle for any patient due to the distance from their home, cost of transportation, additional time off of work, and the number of visits required for trial participation. Bringing trials closer to local communities and rural areas is a critical need to move oncology treatment forward.
The FDA within the US Department of Health and Human Services is actively working on a draft proposal for the decentralization of clinical trials. The goal is to provide some guidance on trial-related tasks that can be done either at a centralized location or at a facility closer to the participant’s home. “Decentralized clinical trials have the potential to expand access to more diverse patient populations and improve trial efficiencies.”19
Navigators Help Identify Eligible Patients, Appropriate Trials, and Overcome Barriers to Help More Patients Enroll in Trials
Nurse navigators are involved in the cancer patient’s care from the initial diagnosis to survivorship. This gives them many opportunities to introduce the idea of a clinical trial to their patients. Having that open conversation with the patient, informing the treating oncologist, and initiating the discussion at tumor board are all ways of closing the gap to enrollment on clinical trials. As navigators, we should have a basic understanding of clinical trials and which ones are available for the specific cancer patient we are navigating. Assessing social or financial barriers is a common task for navigators; adding an additional layer of assessing these ahead of time for clinical trial enrollment would further assist the patient in pursuing the option of enrolling in a trial.
Summary
Clinical trials are an important aspect of cancer care, whether it is participating at time of initial diagnosis, after a recurrence on standard of care, or after a mutation has been recognized. At some or all of these points along the patient’s cancer journey, the option of clinical trials should be brought up for consideration. Importantly, this needs to start at the beginning of their journey, and not only as a “last-ditch effort.”
Nurse navigators need to be informed on the basics of clinical trials so the process can be explained upfront to our patients, the myths can be dispelled, and patients can consider options to access cutting-edge treatments throughout their journey. Bringing this up in the early stages also allows us, as nurse navigators, to anticipate the barriers a patient will have to conquer in order to participate. Let’s give them every opportunity to make it into survivorship.
References
- American Cancer Society. Glossary: Definitions & Phonetic Pronunciations. www.cancer.org/cancer/understanding-cancer/glossary.html?term=clinical+trials. Accessed May 11, 2023.
- Johns Hopkins Medicine. Clinical Research: What Is It? www.hopkinsmedicine.org/research/understanding-clinical-trials/clinical-research-what-is- it.html. Accessed May 8, 2023.
- National Institute on Aging. What Are Clinical Trials and Studies? www.nia.nih.gov/health/what-are-clinical-trials-and-studies. Accessed May 2, 2023.
- Cavazzoni P, Anagnostiadis E, Lolic M. FDA 2020 Drug Trials Snapshots Summary Report. www.fda.gov/media/145718/download. February 2021.
- United States Census Bureau. Quick Facts United States. www.census.gov/quickfacts/fact/table/US/PST045221. Accessed May 2, 2023.
- Holmes DR, Major J, Lyonga DE, et al. Increasing minority patient participation in cancer clinical trials using oncology nurse navigation. Am J Surg. 2012;203:415-422.
- Bloomberg Law. Diversity in Clinical Trials at FDA Gets a Boost From New Law. https://news.bloomberglaw.com/pharma-and-life-sciences/diversity-in-clinical-trials-at-fda-gets-a-boost-from-new-law. Published January 19, 2023. Accessed May 11, 2023.
- Eskander MF, Gil L, Beal EW, et al. Access denied: inequities in clinical trial enrollment for pancreatic cancer. Ann Surg Oncol. 2022;29:1271-1277.
- Center for Information and Study on Clinical Research Participation. CISCRP Perceptions and Insights Study 2021. www.ciscrp.org/wp-content/uploads/2021/11/2021-PI-Deciding-to-Participate-Report-04NOV2021-FI NAL.pdf. Accessed May 3, 2023.
- Li BT, Daly B, Gospodarowicz M, et al. Reimagining patient-centric cancer clinical trials: a multi-stakeholder international coalition. Nat Med. 2022;28:620-626.
- National Institutes of Health. The Basics. www.nih.gov/health-information/nih-clinical-research-trials-you/basics. Accessed May 11, 2023.
- National Cancer Institute. Dictionary of Cancer Terms. www.cancer.gov/publications/dictionaries/cancer-terms/def/randomized-clinical-trial. Accessed May 9, 2023.
- US Food & Drug Administration. The Drug Development Process Step 3: Clinical Research. www.fda.gov/patients/drug-development-process/step-3-clinical-research. Updated January 2018. Accessed May 11, 2023.
- National Comprehensive Cancer Network. Policy Priority: Ensure patient accessibility to guideline-supported care. www.nccn.org/business-policy/policy-and-advocacy-program/policy-priority-ensure-patient-accessibility. Accessed May 5, 2023.
- National Cancer Institute. Insurance Coverage and Clinical Trials. www.cancer.gov/about-cancer/treatment/clinical-trials/paying/insurance. Accessed May 11, 2023.
- US Department of Health and Human Services. Decentralized Clinical Trials for Drugs, Biological Products, and Devices. www.fda.gov/media/167696/download. Accessed May 11, 2023.
- Beck JT, Rammage M, Jackson GP, et al. Artificial intelligence tool for optimizing eligibility screening for clinical trials in a large community cancer center. JCO Clin Cancer Inform. 2020;4:50-59.
- Haddad T, Helgeson JM, Pomerleau KE, et al. Accuracy of an artificial intelligence system for cancer clinical trial eligibility screening: retrospective pilot study. JMIR Med Inform. 2021;9:e27767.
- US Food & Drug Administration. Decentralized Clinical Trials for Drugs, Biological Products, and Devices. Guidance for Industry, Investigators, and Other Stakeholders. www.fda.gov/media/167696/download. May 2023.



