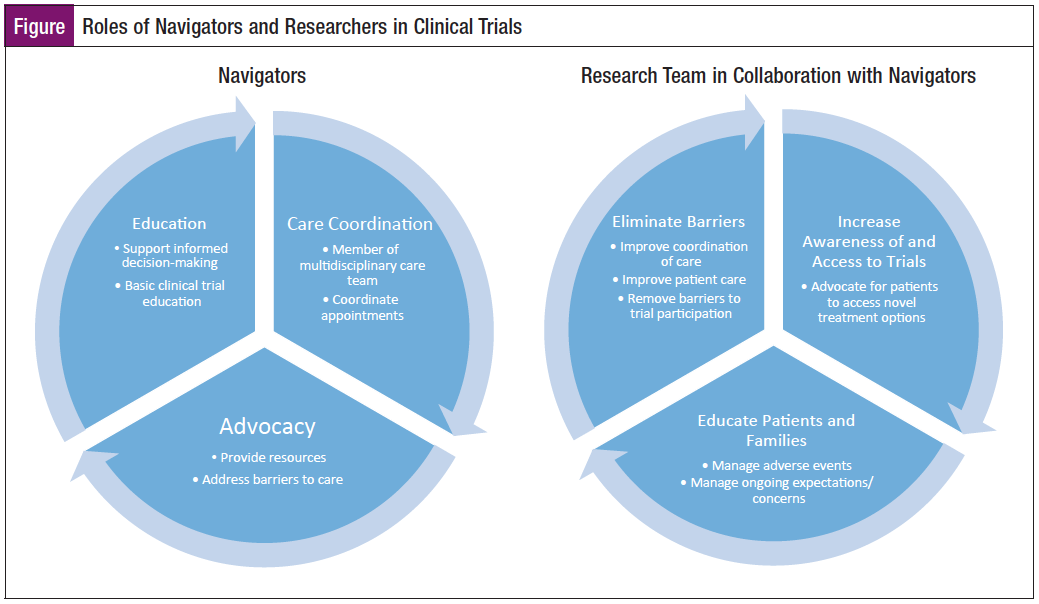
As navigators, we strive to bring the highest quality of care to our patients by including clinical research nurses as critical members of the team. Although there are clear role distinctions between navigators and clinical research nurses, they work together as part of a cross-functional team. Both navigators and researchers work toward the goal of empowering patients with knowledge about all their treatment options, including novel treatments on clinical trials (Figure).
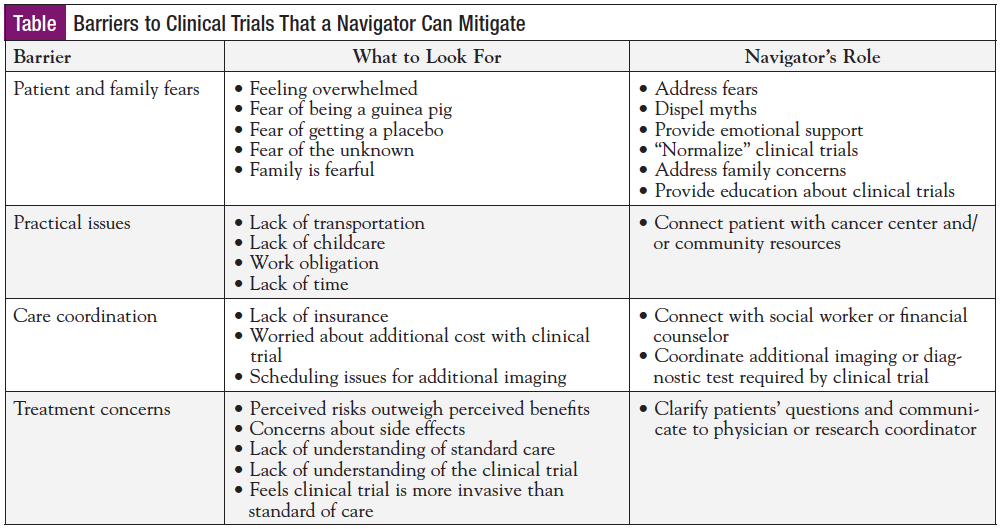
Navigators coordinate care, continuously address patient needs, and remove barriers to ensure access to appropriate specialists, tests, and novel treatments. They facilitate communications with our healthcare team and empower patients with knowledge to support their personal healthcare decisions. It is imperative that nurse and patient navigators are equipped to support access to clinical trials, dispel myths about study participation, and educate patients about their treatment options to include clinical trials (Table). It is vital to provide the resources patients need to access newer treatments by way of clinical trials. By supporting clinical trials to the care team and patients, navigators build and utilize trusting relationships with patients to navigate them toward informed decisions about clinical trials. Navigators serve as the link between patients and their access and awareness of clinical trial options for high-quality oncology care.
Research nurses often remain in close communication with navigators throughout the diagnosis, staging, treatment, and follow-up phases of patient care. Clinical research nurses are often working under titles such as Research Coordinator, Research Nurse, Clinical Trials Associate, or other similar designations. Patient referrals from navigators in the earliest stages of patient care are an incredible resource to help identify individuals who are interested and eligible for screening and participation in clinical trials. Once potential patients are identified, it is the responsibility of the research nurse to provide guidance about the complexities of study participation, which can vary across the disease continuum. The main responsibility of research nurses and staff managing clinical trial protocols is to identify and enroll patients into clinical trials, manage their care according to protocol specifications, and collect/report accurate data to the care team, sponsors, and regulatory officials.
Tips and Talking Points
Initiating a discussion about clinical trials can present a challenge. Below, we offer practical suggestions to employ in daily discussions with patients and the care team that may improve access and participation in clinical trials.
To Introduce Clinical Trials
- “Have you talked to your doctor about clinical trial options?”
- “We offer many clinical trials; let me connect you with my research nurse colleagues to see what options you can consider.”
To Address Common Myths/Misconceptions
Receiving a placebo or being undertreated
- “Our center ensures that all oncology patients receive medical treatment for their disease. Participating in a clinical trial ensures you receive the best standard-of-care treatment, with the addition of a new investigational agent.”
Considered part of a “human experiment” or “guinea pig”
- “We follow strict federal, state, and institutional policies to ensure all clinical trials are conducted ethically. Long gone are the days of unregulated experiments.”
Research as a last resort
- “There are many exciting clinical trials available to patients with all stages and types of disease. They may allow you to access additional treatments.”
To Explain the Randomization Process
- “Clinical trials separate patients into groups (“control group” vs “experimental group”) to compare the results of different treatments. Our trial options include studies in which all patients receive standard treatment, regardless of study group. Having the control group ensures that researchers can compare the standard treatment alone against the experimental group receiving standard treatment plus the addition of an investigational agent. You will be randomly assigned to a group—similar to having a baby, you may have a boy or a girl—but you will not know which group until you are enrolled in the trial.”
AONN+ Domain of Coordination of Care/Care Transitions
The role of the navigator in clinical trials is an important aspect of the Academy of Oncology Nurse & Patient Navigators (AONN+) Domain of Coordination of Care/Care Transitions. By collaborating with clinical research teams, navigators can show their value in clinical trials education, which is outlined in the AONN+ Metrics.
The standardized metrics addressing clinical trials are as follows:
- Clinical Trial Education: Number of patients educated about clinical trials by the navigator per month (Measures patient experience and clinical outcomes)
- Clinical Trial Referrals: Number of navigated patients per month referred to clinical trial department (Measures patient experience and clinical outcomes)
Utilizing the AONN+ domains of knowledge in regard to coordination of care, navigators can play an important role in clinical trials by increasing awareness and access to trials, educating patients and families, and eliminating barriers to trial participation.
Resources
National Cancer Institute booklet for patients explaining what a clinical trial is. Taking Part in Cancer Treatment Research Studies. www.cancer.gov/publications/patient-education/cancer-treatment-research-studies
To find a trial: https://clinicaltrials.gov