Multiple myeloma (MM) is a rare blood cancer characterized by bone marrow infiltration of monoclonal plasma cells.1 The malignant plasma cells accumulate in the bone marrow and secrete an immunoglobulin that can usually be detected in the blood and urine. This abnormal accumulation of immunoglobulin, or M-protein, results in dysfunction, causing many patients to begin developing symptoms.1 The primary goal of treatment in patients with MM is to increase survival and maintain a reasonable quality of life despite often debilitating symptoms.2 Treatment options for MM include corticosteroids, chemotherapy such as alkylating agents and anthracyclines, proteasome inhibitors, immunomodulatory drugs, histone deacetylase inhibitors, monoclonal antibodies, chimeric antigen receptor (CAR) T-cell immunotherapies, and, more recently, bispecific antibodies, which have been demonstrated to improve patient outcomes.1 Even with novel treatment advances, many patients with MM will relapse and require changes in treatment regimens, or they may become treatment refractory primarily due to drug resistance.1 The interaction of malignant MM cells in the surrounding bone marrow leads to an immunosuppressive microenvironment which may decrease immunotherapy efficacy.3 Bispecific antibodies are unique antibody constructs that simultaneously bind 2 antigens, typically targeting an antigen on myeloma cells and a molecule on an immune cell. By using the patient’s immune system to destroy the malignant plasma cells, bispecific antibodies offer a promising treatment to overcome immunosuppression for patients with relapsing/refractory MM (RRMM).4
Expanding therapeutic options have improved prognosis and overall survival for patients with MM but have also added complexity to treatment algorithms. This can make it difficult for healthcare professionals, including oncology nurse navigators, to stay up to date on current MM therapy and appropriate patient management considerations. Bispecific antibodies in oncology have novel mechanisms of action (MOAs), clinical safety profiles and adverse event (AE) management, and treatment sequencing2,5,6 and their use can provide healthcare professionals further opportunities to support, engage, and educate patients and their caregivers during their journey with MM.7,8
Overview of the MOA of Bispecific Antibodies and Rationale for Use in Multiple Myeloma
MM is the second most common hematologic cancer and, although there is a better understanding of the disease and development of improved treatments, the median overall survival in patients is still only about 8 to 10 years.1 In an effort to improve clinical outcomes in patients with MM, extensive research has focused on T-cell–directed cytotoxicity on cancer cells through the use of immune checkpoint inhibitors, CAR T-cells, and bispecific antibodies.9 Bispecific antibodies are recombinant molecules which have 2 binding sites directed at different antigens (Figure 1) or 2 different epitopes of 1 antigen simultaneously.2,5,6 In MM treatment, bispecific antibodies target antigens on immune cells such as T-cells or natural killer cells and tumor antigens on malignant plasma cells.5 This bispecific binding results in T-cell activation, T-cell proliferation, and, in MM, plasma cell lysis.2,3 The main targets for most bispecific antibodies in MM are the CD3 receptor; B-cell maturation antigen (BCMA); G protein–coupled receptor, class C, group 5, member D (GPRC5D); and anti–Fc receptor-homolog 5 (anti-FcRH5).10-13
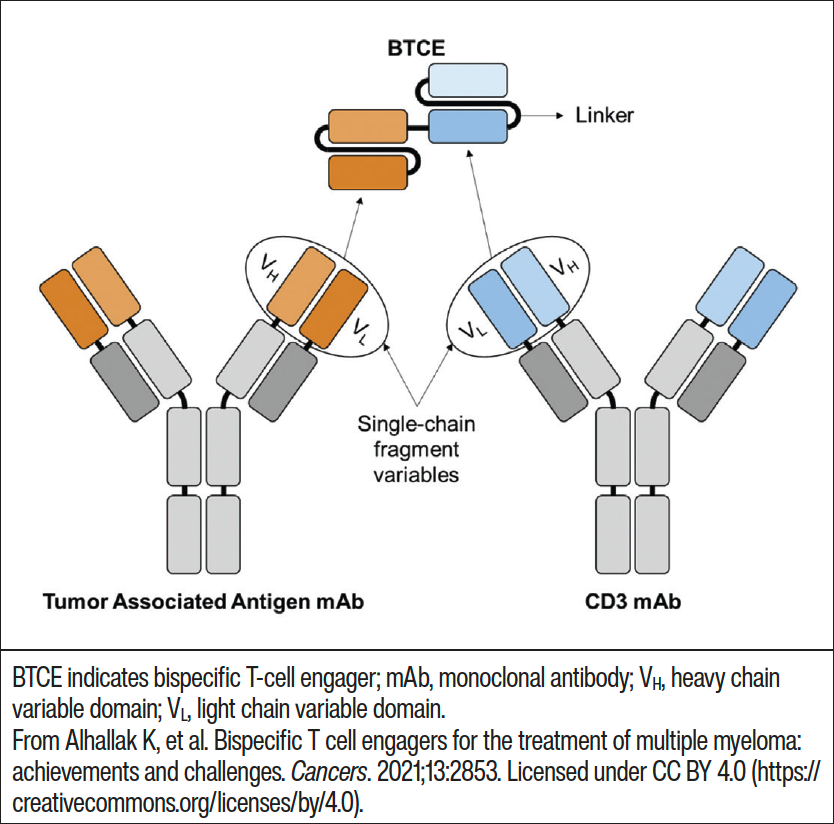
Figure 1. Creation of a T-cell–engaging bispecific antibody using 2 monoclonal antibodies linked together.9
Bispecific antibodies differ from the currently approved monoclonal antibodies as they bind to both the plasma cells and the cytotoxic T-cells found in MM, as opposed to monoclonal antibodies which bind to the same antigen target.10,14 Bispecific antibodies can offer several advantages over monoclonal antibodies. First, they have enhanced cytotoxicity as they are able to direct specific effectors of the immune system to target tumor cells. Because bispecific antibodies interact with 2 different surface antigens, they are also able to offer higher binding specificity compared to monoclonal antibodies.15 Additionally, they may improve treatment efficacy and safety over monoclonal antibodies by focusing the immune activating pharmacologic effects on the tumor area.3 Monoclonal antibodies are often combined with a cytotoxic drug in MM; therefore, the use of a bispecific antibody as opposed to combination therapy can help streamline treatment management, including potentially reducing treatment cost.15
In CAR T-cell therapy, a patient’s T-cells are isolated from the patient’s peripheral blood, modified ex vivo so the T-cells are able to attach to a specific cancer cell antigen, and infused back into the patient to perform their function.10 Although CAR T-cell therapy offers the potential for a one-time treatment, there may be significant administration delays due to manufacturing, including a manufacturing fail rate of about 10%. Because CAR T-cell development is individualized for each patient, there may also be significant financial burden. Bispecific antibodies as an off-the-shelf treatment have the advantage (over CAR T-cell therapy) of having more reliable manufacturing.16 Bispecific antibodies may also have a lower risk of treatment-related AEs (TRAEs), such as cytokine release syndrome (CRS) and neurotoxicity, and a lower risk of target antigen loss.16 The treatment response rate, however, is generally lower with bispecific antibody use when compared with CAR T-cell therapy.16
Bispecific Antibodies Approved for Use in the United States
Bispecific antibodies are being investigated in a wide range of therapeutic areas. Currently there are 9 bispecific antibodies which are approved worldwide, with 7 approved in the United States by the Food and Drug Administration (FDA).17,18 Blinatumomab was approved in 2014 for the treatment of relapsed or refractory precursor B-cell acute lymphoblastic leukemia (ALL) and targets CD3 and CD19.6 Further approval was given in 2017 for Philadelphia-positive ALL and for patients with minimal residual disease (MRD)-positive ALL in 2018.6 This further approval was based upon the results of the phase 3 TOWER clinical trial, which found the median overall survival in patients treated with blinatumomab was nearly twice as long as patients treated with common chemotherapy used in relapsed or refractory B-cell ALL (7.7 months vs 4 months; hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.55-0.93; P = .01).19 Emicizumab is another FDA-approved bispecific antibody that targets factors FIXa/FX and was approved in 2017 for the treatment of bleeding due to hemophilia.6 This approval was based on the results of 2 clinical trials: HAVEN 1 in adults and HAVEN 2 in children.20 In 2018, emicizumab was approved for patients with hemophilia A with or without factor-VIII inhibitors based upon the results of the HAVEN 3 and HAVEN 4 clinical trials.21 In 2021, amivantamab, which targets epidermal growth factor (EGFR) and MET receptors, was approved to treat adult patients with locally advanced or metastatic non–small-cell lung cancer (NSCLC) harboring the EGFR exon 20 mutation who had disease progression after platinum-based chemotherapy.22 This approval was based upon the positive overall response rate (ORR, 40%) and median duration of response (11.1 months) results of the CHRYSALIS clinical trial, which included patients with advanced NSCLC with EGFR exon 20 insertion.22 Teclistamab, tebentafusp, and faricimab are bispecific antibodies that were all approved in 2022. Teclistamab, which is approved in RRMM, is a T-cell–redirecting bispecific antibody which targets CD3 expressed on the T-cell surface and BCMA on the myeloma cell surface.23 Tebentafusp targets melanocyte differentiation antigen polypeptide glycoprotein 100 and CD3 and is approved to treat unresectable or metastatic uveal melanoma.6 In an open-label, phase-3 study, it outperformed single-agent dacarbazine, ipilimumab, and pembrolizumab in patients with newly diagnosed metastatic uveal melanoma when overall survival was evaluated; overall survival at 1 year was 73% with tebentafusp versus 59% in the control group (HR, 0.51; 95% CI, 0.37-0.71; P <.001) in the intention-to-treat population.24 Faricimab is used to treat wet age-related macular degeneration (AMD) and diabetic macular edema (DME).25 Faricimab targets and inhibits angiopoietin-2 and vascular endothelial growth factor-A pathways, which destabilize blood vessels contributing to vision loss. The approval for faricimab was based upon the results from the phase 3 TENAYA and LUCERNE clinical trials for wet AMD and the phase 3 YOSEMITE and RHINE clinical trials for DME.25 All trials found that faricimab was noninferior to aflibercept with comparable incidence of ocular AEs.25 In late 2022, mosunetuzumab gained FDA approval for the treatment of adults with relapsed or refractory follicular lymphoma after 2 or more prior lines of systemic therapy.18 The approval was granted based upon data from the phase 2 GO29781 study (NCT02500407), which demonstrated high and durable response rates in trial participants.18 Other bispecific antibodies, which are approved outside the United States, include cadonilimab (cervical cancer treatment in China) and ozoralizumab (inflammatory diseases in Japan).6
Teclistamab: FDA-Approved Bispecific Antibody for Multiple Myeloma
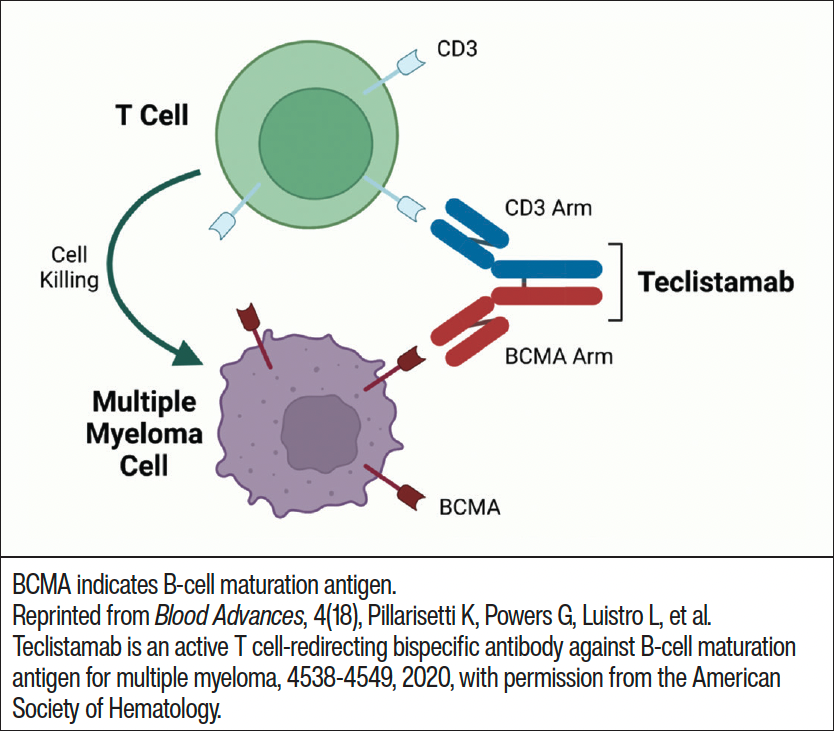
Although there are several FDA-approved bispecific antibodies for various therapeutic indications, teclistamab is the only bispecific antibody approved for treatment of MM. Teclistamab forms a bridge between myeloma cells and T-cells, enabling the T-cell to recognize the cancer cell and trigger a series of events that lead to myeloma cell death (Figure 2).26 The phase 1/2 MajesTEC-1 trial evaluated the safety and efficacy of teclistamab in 165 patients with RRMM who had previous exposure to an immunomodulatory drug, anti-CD38 antibody, and proteasome inhibitor.27 The primary study end point was ORR, and the median follow-up was 14.1 months. Study participants received 1.5 mg/kg teclistamab subcutaneously (SC) once a week after step-up doses of 0.06 mg/kg and 0.3 mg/kg. A median of 5 prior lines of therapy had been given to patients, and 77.8% had triple-class refractory disease. The median time to first response was 1.2 months with an ORR of 63.0%.27 A complete response or better (≥CR) was experienced by 65 patients, with 44 patients having no MRD. For patients with a ≥CR, the MRD-negativity rate was 46%.27 The median progression-free survival was 11.3 months while the median duration of response was 18.4 months.27 All patients experienced ≥1 AE with 94.5% of patients experiencing a grade 3 or 4 AE. The most common nonhematologic AEs experienced by study participants included injection-site reaction, fatigue, pyrexia, CRS, upper respiratory tract infection, pneumonia, nausea, diarrhea, and musculoskeletal pain.27 The most common hematologic AEs were neutropenia (70.9%, 64.2% grade 3-4), anemia (52.1%, 37% grade 3-4), lymphopenia (34.5%, 32.7% grade 3-4), and thrombocytopenia (40%, 21.2% grade 3-4).27 A total of 76.4% of patients experienced infections, with 44.8% of these infections being grade 3 or 4.27 CRS occurred in 72.1% of patients, most were grade 1 or 2 and occurred after step-up and cycle 1 doses. Neurotoxic events occurred in 24 patients, including 5 patients who experienced immune effector cell–associated neurotoxicity syndrome (ICANS).27 The toxic effects that were associated with T-cell redirections were primarily grade 1 or 2.27
In the ongoing teclistamab clinical trial program, the MajesTEC-2 trial (NCT04722146) is investigating teclistamab in combination with SC daratumumab and lenalidomide in patients with MM at 2 doses: 0.72 mg/kg or 1.5 mg/kg weekly.28 Daratumumab and lenalidomide plus dexamethasone have already been approved for treatment in MM and may enhance the function of teclistamab. Initial safety and efficacy results found that the combination was well tolerated with a safety profile matching teclistamab or daratumumab and lenalidomide individually.28 The median time to response was 1 month.29 The ORR was 93.5% with very good partial responses or better in 90.3% of patients and 54.8% achieving a ≥CR.29 The phase 3 MajesTEC-7 (NCT05552222) study will compare this drug combination to daratumumab, teclistamab, and dexamethasone in patients with newly diagnosed MM ineligible or not intended for autologous stem-cell transplant as initial treatment.28
At the recommended dose, teclistamab demonstrated durable responses which were well tolerated.27 Based upon the results of the MajesTEC-1 clinical trial, the FDA granted accelerated approval of teclistamab on October 25, 2022, for adult patients with RRMM who have been treated with ≥4 prior lines of therapy, including an anti-CD38 monoclonal antibody, an immunomodulatory agent, and a proteasome inhibitor.23
Bispecific Antibodies Under Current Investigation in Multiple Myeloma
Although teclistamab is the only currently approved bispecific antibody for MM, there are several that are being investigated in a number of clinical trials.
Elranatamab
Elranatamab, a bispecific antibody that targets both CD3-expressing T-cells and BCMA-expressing MM cells, is being evaluated in the MagnetisMM clinical trial program.30 MagnetisMM-3 (NCT04649359) is an open-label, multicenter, non-randomized, phase 2 clinical trial investigating the safety and efficacy of elranatamab monotherapy in patients with RRMM.30 Patients were required to be refractory to ≥1 proteasome inhibitors, 1 immunomodulatory drug, and 1 anti-CD38 antibody and were assigned to 2 cohorts: patients who were naïve to BCMA-directed therapies (cohort A) or those who were previously treated with BCMA-directed antibody–drug conjugates or CAR T-cells (cohort B). The study’s primary end point was ORR. A 2-step priming dose (12 mg and 32 mg) was administered the first week, then patients were given elranatamab 76 mg SC every week on a 28-day cycle with dose modifications allowed if toxicity was experienced. From this initial evaluation, it was found that elranatamab was well tolerated at the 2-step-up priming regimen and 76-mg dose with no grade 3 or greater CRS or ICANS observed.30 Further study evaluation examined 123 patients who were in cohort A.31 Median duration of treatment was 5.3 months (range, 0.03-16.1) with 51.2% of patients still receiving elranatamab at the cutoff for data collection. Progressive disease (32.5%) and AEs (7.3%) were the primary reasons given for permanent treatment discontinuation. The ORR was 61.0% (95% CI, 51.8-69.6) and the median time to response was 1.2 months. All patients experienced any-grade TRAEs, while 74.8% experienced grade 3 or 4 TRAEs. ICANS and CRS events were all grade 1 or 2 with no patients permanently discontinuing treatment due to these events.31 This further evaluation found that elranatamab continued to be efficacious with a tolerable safety profile. Additionally, MagnetisMM-4 is a phase 1B/2 umbrella study (NCT05090566), currently enrolling patients, which aims to evaluate elranatamab in combination with other anticancer treatments in patients with MM.32 The phase 3 MagnetisMM-5 study (NCT05020236) is evaluating the efficacy and safety of SC elranatamab monotherapy and in combination with daratumumab in patients with RRMM.33
Talquetamab
Talquetamab is the first bispecific antibody against both CD3 and GPRC5D, which is an orphan receptor with limited expression in normal human cells but is highly expressed in malignant plasma cells found in MM.11,13 MonumenTAL-1 (NCT03399799) is a phase 1 clinical trial evaluating talquetamab in patients with RRMM. Eligible patients had RRMM or were intolerant to standard therapies; prior BCMA-directed therapy was allowed. The study primary objective was to identify recommended doses and to assess safety and tolerability at the recommended dose.11 Two dosing regimens were determined to be efficacious and safe: 405 μg/kg SC once weekly (70% response rate at median 11.7-month follow-up) and 800 μg/kg SC every other week (64% response rate at median 4.2-month follow-up).11,13 Severe CRS was mitigated by step-up dosing and premedicating during the step-up dosing and at the first full dose.11 The most common TRAEs were CRS, skin-related events, and dysgeusia, but all were grade 1 or 2 with 1 dose-limiting toxic grade 3 rash observed in a patient receiving the 800-μg dose of talquetamab.11
Key Ongoing Trial Data Highlights:
- Imvotamab is a novel IgM CD20 x CD3 T-cell–engaging bispecific antibody. Imvotamab is currently being investigated in a phase 1/2 study (NCT04082936) with results demonstrating that monotherapy had durable responses along with a favorable safety profile and tolerability up to 1000-mg dosing. Imvotamab enables irreversible target cell binding even at low CD20 levels with biological activity starting at 10 mg. This results in T-cell–dependent and complement-dependent mechanisms of cytotoxicity with minimal cytokine release. Dose titrating is supported as a safe and efficacious means to administer imvotamab.34
- REGN5458 is a BCMA x CD3 bispecific antibody which induces T-cell cytotoxicity on MM plasma cells. In a phase 1/2 trial (NCT03761108), patients with RRMM who were double or triple refractory or intolerant to systemic therapy were treated with doses ranging from 3 to 800 mg. Patients had a median of 5 prior lines of therapy, with a range from 2 to 17.35 At all dose levels responses were observed with an ORR of 50.7%; among responders, 86.5% had at least a very good partial response and 43.2% had a complete or stringent complete response. The safety profile was tolerable with no treatment discontinuation due to CRS.35 Based upon these positive findings, a phase 1b study was designed to explore the benefits of combining REGN5458 with other anticancer agents: daratumumab plus dexamethasone, carfilzomib plus dexamethasone, lenalidomide plus dexamethasone, and bortezomib plus dexamethasone. The primary end points are safety and tolerability of the combinations and TRAEs for each study’s cohorts.36 A phase 2 study of REGN5458 monotherapy is assessing the safety, tolerability, pharmacokinetics, and preliminary efficacy in patients with newly diagnosed MM who are eligible or ineligible for high-dose chemotherapy and autologous stem-cell transplantation.35
- Cevostamab is an FcRH5 and CD3 bispecific antibody which facilitates T-cell–directed killing of myeloma cells.12 In the ongoing phase 1 clinical trial (NCT03275103), cevostamab monotherapy in patients with heavily pretreated RRMM demonstrated near ubiquitous FcRH5 expression on myeloma cells along with manageable safety and promising activity when given every 3 weeks for up to 17 cycles.12 There was a target dose–dependent increase in ORR without an increase in CRS rate. In an updated report of patients who completed 17 cycles of cevostamab and stopped treatment, the early duration-of-response data were presented.37 All patients had no available, appropriate, or tolerable established therapy. From the data evaluation, all patients maintained durable responses ≥6 months after completion of 17 treatment cycles.37
- RG6234 is a GPRC5D x CD3 T-cell–engaging bispecific antibody. GPRC5D is overexpressed on MM cells and RG6234 binds to both GPRC5D and CD3, leading to T-cell–directed tumor-cell killing. A phase 1 clinical trial (NCT04557150) demonstrated an ORR with intravenous (IV) administration of RG6234 of 71.4% and an ORR with SC administration of 60.4%.38
Bispecific Antibody Therapy Side Effects and Management
With the increasing adoption of bispecific antibodies into clinical practice for the treatment of MM, appropriate side-effect management is crucial to optimizing patient care. Common side effects seen in clinical trials include CRS, neurotoxicity, infections, hematologic TRAEs, and dermatologic TRAEs.4 In a pooled analysis of 10 clinical trials with a total of 790 patients with MM treated with a bispecific antibody (73% with a BCMA agent), 100% of patients experienced a TRAE, highlighting the need for appropriate management strategies.39 Although serious TRAEs occur in some patients receiving bispecific antibodies, most can be managed effectively without treatment interruption or discontinuation.
CRS and Neurotoxicity
CRS is an acute systemic inflammatory syndrome which involves activation of T-cells and other immune effector cells with significant cytokine release.4 CRS severity is a result of a combination of factors including the patient’s type of underlying malignancy, disease burden, and immunotherapy type and dose.4 Although fever is the hallmark symptom of CRS, other symptoms which may develop include dyspnea, pulmonary edema, chills, fatigue, malaise, hypotension, tachycardia, troponin leak, nausea, and/or rash.4 CRS occurs in over 80% of patients with bispecific antibody treatment; however, it is typically manageable, with less than 10% of patients experiencing grade 3 or 4 symptoms.4 The median time to onset and median duration of CRS in clinical trials ranged from 12 to 48 hours, generally with a quicker onset with IV administration (24 hours) versus SC administration (48 hours).4 Early recognition and treatment of CRS is important to prevent serious complications. Step-up dosing requires hospitalization for monitoring of vital signs, for signs of infections, and for laboratory monitoring.40 If a patient develops low-grade CRS, treatment is recommended with tocilizumab and dexamethasone along with low-flow oxygen by nasal cannula and single vasopressor.8,40 However, due to recent shortages of tocilizumab, siltuximab and anakinra are being evaluated for use in mitigating CRS.
Neurotoxicity is experienced by <30% of patients treated with immune therapy, with <10% of patients having grade 3-4 symptoms.4 Neurotoxicity can occur between 2 and 30 days after treatment but can happen past 30 days posttreatment.40 Symptoms of neurotoxicity generally last 3 to 14 days and include confusion, delirium, aphasia, tremor, loss of coordination, Parkinson-like symptoms, and seizures. Neurotoxicity may occur in the presence of CRS and, if so, will resolve with CRS treatment.4 Of note, most cases in clinical trials have been reversible. Supportive measures for neurotoxicity include tocilizumab, dexamethasone, and/or levetiracetam.8
Hematologic Adverse Events
Hematologic side effects also occur with bispecific antibody use and include low white blood cell count, low platelets, and anemia. Hematologic side effects across clinical trials have shown an incidence of anemia of 21% to 42% any grade and 17% to 42% grade ≥3, an incidence of neutropenia of 16% to 57% any grade and 14% to 53% grade ≥3, an incidence of lymphopenia of 15% to 40% any grade and 12% to 36% grade ≥3, an incidence of leukopenia of 23% to 32% any grade and 14% to 16% grade ≥3, and an incidence of thrombocytopenia of 8% to 40% any grade and 6% to 23% grade ≥3.4 Low blood cell counts are more common in the first few treatment cycles and can be seen regardless of patient response to therapy. They may also be caused by CRS. Available data on hematologic side effects are currently lacking regarding timing, duration, mechanisms, or dose–response relationship.
Infections
Infection is relatively common with bispecific antibody use, with one major concern being B-cell depletion from treatment targeting B-cell markers.8 This depletion may inhibit the ability to produce a neutralizing IgG response leading to an immunodeficient state. Up to 33% of patients in clinical trials have experienced serious infections requiring hospitalization and IV antibody treatment. There is an increased risk of serious COVID-19 infection despite vaccination status in patients with MM treated with bispecific antibodies.40 In a pooled analysis study, all-grade COVID-19 occurred in 15% of patients, with grade 3-4 COVID-19 occurring in 11% of patients. This is of particular concern for BCMA-treated patients as they may be predisposed to developing hypogammaglobulinemia, they may lack a good vaccine response, and they are predisposed to developing complications from infection. Nirmatrelvir with ritonavir (Paxlovid) should be started immediately if COVID-19 is diagnosed as Paxlovid must be started within 5 days of the start of symptoms.40 Infection-mitigating measures such as avoiding crowds, handwashing, IV immune globulin for hypogammaglobulinemia, current immunizations, and COVID-19 prevention (vaccinations, appropriate masking, ventilation) are strongly encouraged.
Dermatologic Adverse Events
Dermatologic toxicities also occur with bispecific antibody therapy. Injection-site reactions have been reported in approximately 25% of patients, typically occurring in the first treatment cycle.40 Treatment for injection-site reactions is with oral histamine and topical steroids.40 With anti-GPRC5D treatment, skin toxicities can be dramatic.4,40 Nail thinning, peeling, and lifting from the nail bed have been observed. Treatment is with over-the-counter nail hardener, topical triamcinolone ointment if nails are inflamed, and topical vitamin E ointment.40 Palmar plantar desquamation can also occur in the first treatment cycle. It is typically painless and is treated with ammonium lactate lotion.40 Finally, the oral cavity can also be affected by bispecific antibody use, leading to dry mouth, altered taste, and difficulty swallowing with possible weight loss in affected patients.40 These symptoms can be managed with dose adjustments and dry mouth sprays and rinses.
The Nurse Navigator Role in Multiple Myeloma
Nursing education, management, and support have always been integral for optimal patient care. While the management and care of patients with MM requires the skills and talents of a multidisciplinary team, nurse navigators have vital roles within the team to act as patient advocates; they support patients, their families, and caregivers and help address barriers to timely and appropriate cancer care.41 A cancer diagnosis can be overwhelming, and management is often complex where many patients may have limited knowledge and/or capability on how to navigate through the medical, psychosocial, and financial aspects of their cancer journey.41 This may lead to treatment delays, decreased patient satisfaction, and suboptimal quality of care. Nurse navigators empower patients via education so they and their families can make informed decisions in order to participate in healthcare planning.7 In addition, they can facilitate communication between patients and other members of the multidisciplinary healthcare team and provide healthcare and educational resources.41 By building rapport with patients, nurse navigators can gain an understanding of each person’s situation to allow for the identification of barriers to care throughout the cancer continuum and implement strategies to mitigate these barriers as necessary.41
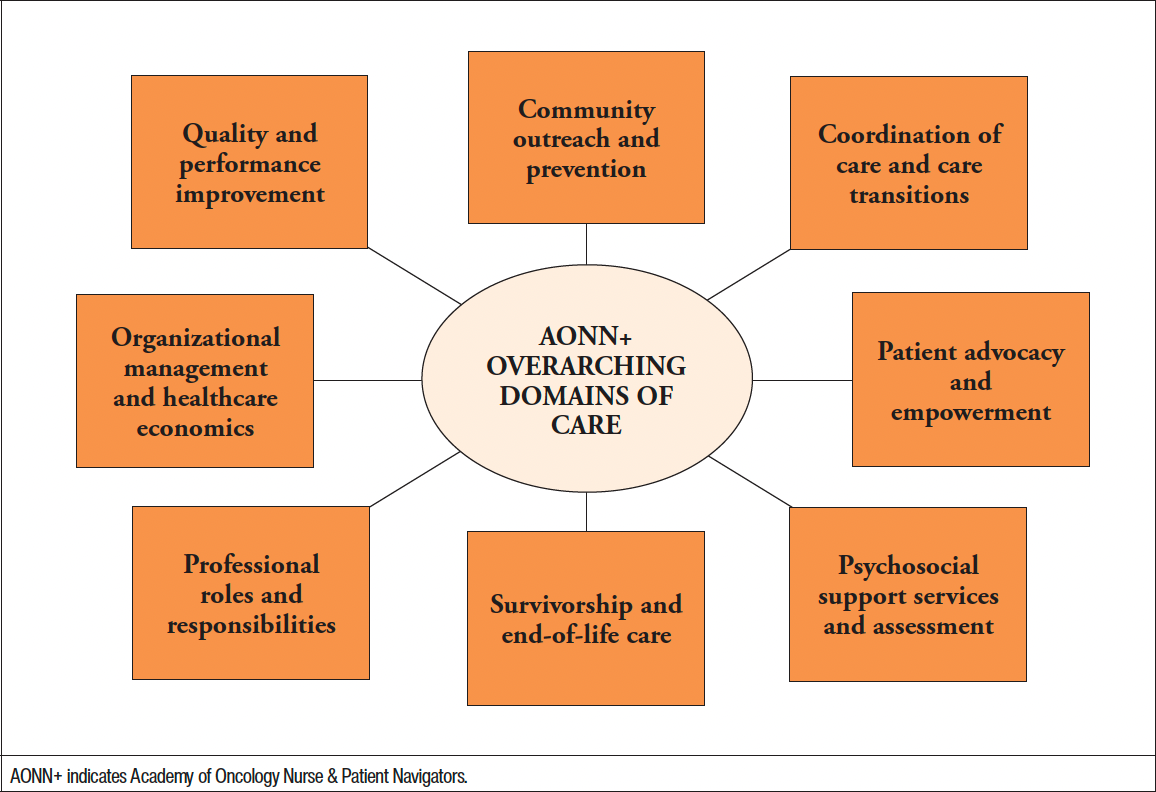
The Academy of Oncology Nurse & Patient Navigators (AONN+) has identified a number of overarching domains of care which nurse navigators should demonstrate knowledge of in order to provide quality patient-centered care (Figure 3).7
Additionally, the Oncology Nursing Society has established 4 broad core competencies for the role of a nurse navigator. These competencies are described under 4 categories: education, coordination of care, overcoming healthcare system barriers, and professional role.7 Although the core competencies remain the same across cancer type, hematologic malignancies offer unique challenges for patients as they are a diverse group of cancers that have distinct disease courses, are associated with different prognosis and survival, and have distinct treatment strategies.7 Education is a fundamental role for the nurse navigator, as understanding medical jargon can be difficult for patients and their families. Nurse navigators play a central role in explaining medical terminology and educating patients on treatment plans; therefore, nurse navigators need to be knowledgeable on the ever-evolving and complex treatment landscape, especially in the field of oncology. In terms of clinical support, nurse navigators help patients recognize and identify treatment-related side effects and encourage them to report these to the healthcare team.7 They can also empower patients by educating them on how to take an active role in self-care. An understanding of the clinical trial process along with risks and benefits of participation in a clinical trial is necessary to keep patients informed of treatment opportunities and to gauge patient receptivity to participation.7 Psychosocial distress is experienced by 50% to 90% of patients with cancer. Uncertainty, fear of cancer recurrence, a sense of vulnerability, and consequences of disease on employment, healthcare, social life, and parenting all cause emotional distress and may decrease treatment adherence, health-related quality of life, and lead to adverse survival outcomes.7 Nurse navigators, through monitoring patients’ psychosocial needs, can help validate fears and concerns and educate patients on available resources, such as support groups or access to counselors.7 Many patients are finding an improved life expectancy with treatments which have given durable remissions. This survivorship comes with economic, healthcare, and medical consequences. Nurse navigators can help these patients by empowering them to manage expectations and by coordinating follow-up care.7
Nurse Navigator Considerations for Managing Patients Receiving Bispecific Antibodies
MM is a challenging disease to treat and manage. Providing tailored disease and treatment information along with information on testing, available treatment options, side effects, and AEs to watch for and report on is a key role for the nurse navigator.8 Patients receiving bispecific antibodies have special nursing assessment needs, and monitoring is focused on the potential for side effects.42 Nurse navigators should report alterations in vitals or laboratory results to the prescriber.42 If applicable, the Risk Evaluation and Mitigation Strategy (REMS) for the bispecific antibody should be reviewed. As with idecabtagene vicleucel and ciltacabtagene autoleucel, the 2 CAR T-cell therapies approved for RRMM, teclistamab is only available through the TECVAYLI™ REMS, due to the risk of CRS and neurologic toxicity.23
Specific considerations for nurse navigators for the care of patients receiving bispecific antibodies include42,43:
- Educate staff and patients on bispecific antibody administration, toxicity management, and what to do about medical emergencies
- Review the patient care plan with patients and their caregivers
- Determine if patients have adequate support during treatment such as nursing services, social workers, and access to transportation
- Confirm patient coverage for the drug by calling the payer or submitting benefit verification
- Confirm coverage for any necessary home healthcare, durable medical equipment, or other medical home needs for patients transitioning to outpatient services
- Ensure multidisciplinary coordination and communication when a patient is transitioning to outpatient services and that the home infusion company is equipped to administer the patient’s bispecific antibody
- Check for and complete the requirements for prior authorization
- Provide patients and their caregivers with financial assistance resources from charities, pharmaceutical companies, or applicable foundations
- Ensure that caregivers have provider contact information, clear instructions on what to do in a medical emergency, and understand the complications of bispecific antibody treatment
- Coordinate infusion appointments including ordering premedications, setup of continuous infusions, and ensure that medication is available (and on hand) to treat potential CRS
- Establish patient follow-up schedules
- Monitor patient well-being before, during, and after treatment and incorporate social work support if necessary.
In a 2020 survey of 129 multidisciplinary cancer provider experiences with bispecific antibodies, the resources that providers desired were identified. Care coordinators and nurse navigators were among the disciplines included in the surveyed providers. Educational resources for patients and caregivers on transitioning from inpatient to outpatient care were deemed beneficial by 82% of respondents, including information on how to address problems that may occur with outpatient administration.44 Peer support services for patients were thought to be of value by 70% of respondents.44 Other desired resources include an in-house or on-site expert, either someone within the organization or from the drug manufacturer, and a list of home health companies or pharmacies familiar with bispecific antibodies. Finally, written guidelines, best practices, and care recommendations were desired by 86% of respondents.44
Conclusion
Bispecific antibodies have unique MOAs, safety profiles, and toxicity management considerations, and have been approved in several different therapeutic categories, including cancer. These therapies offer patients and physicians a broader choice of efficacious treatment options. Teclistamab is currently the only FDA-approved bispecific antibody for patients with RRMM; however, other agents are actively being investigated.
Nurse navigators play a vital role for patients with MM treated with bispecific antibodies. As such, it will remain important for them to stay current with the new and emerging therapies still in clinical trials. As advocates for patients with MM, by staying abreast of these therapies nurse navigators will be poised to support patients and families through their cancer journey by giving them optimal outcomes and quality of life.
References
- Pinto V, Bergantim R, Caires HR, et al. Multiple myeloma: available therapies and causes of drug resistance. Cancers (Basel). 2020;12:407.
- Cipkar C, Chen C, Trudel S. Antibodies and bispecifics for multiple myeloma: effective effector therapy. Hematology Am Soc Hematol Educ Program. 2022;2022:163-172.
- Dahlen E, Veitonmaki N, Norlen P. Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccines Immunother. 2018;6:3-17.
- Lancman G, Sastow DL, Cho HJ, et al. Bispecific antibodies in multiple myeloma: present and future. Blood Cancer Discov. 2021;2:423-433.
- Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616.
- Wei J, Yang Y, Wang G, Liu M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol. 2022;13:1035276.
- JONS. Role of oncology nurse navigators in hematologic malignancies: patient education and empowerment. www.jons-online.com/issues/2016/october-2016-vol-7-no-9?view=article&artid=2728:role-of-oncology-nurse-navigators-in-hematologic-malignancies-patient-education-and-empowerment#:~:text=ONNs%20play%20a%20pivotal%20role. Accessed February 10, 2023.
- JONS. Optimizing bispecific antibody therapy in multiple myeloma: a resource guide for nurse and patient navigators. www.jons-online.com/special-issues-and-supplements/2022/optimizing-bispecific-antibody-therapy-in-multiple-myeloma-a-resource-guide-for-nurse-and-patient-navigators. Accessed February 10, 2023.
- Alhallak K, Sun J, Jeske A, et al. Bispecific T cell engagers for the treatment of multiple myeloma: achievements and challenges. Cancers (Basel). 2021;13:2853.
- Catamero D, Richards T, Faiman B. A focus on CAR T-cell therapy and bispecific antibodies in multiple myeloma. J Adv Pract Oncol. 2022;13(suppl 4):31-43.
- Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387:2232-2244.
- Trudel S, Cohen AD, Krishnan AY, et al. Cevostamab monotherapy continues to show clinically meaningful activity and manageable safety in patients with heavily pre-treated relapsed/refractory multiple myeloma (RRMM): updated results from an ongoing phase I study. Blood. 2021;138(suppl 1):157-157.
- Minnema MC, Krishnan AY, Berdeja JG, et al. Efficacy and safety of talquetamab, a G protein-coupled receptor family C group 5 member D x CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM): updated results from MonumenTAL-1. J Clin Oncol. 2022;40(16 suppl):8015-8015.
- Genentech. The evolution of bispecific antibodies. www.gene.com/stories/the-evolution-of-bispecific-antibodies. Accessed February 10 , 2023.
- Sedykh SE, Prinz VV, Buneva VN, Nevinsky GA. Bispecific antibodies: design, therapy, perspectives. Drug Des Devel Ther. 2018;12:195-208.
- Cho SF, Yeh TJ, Anderson KC, Tai YT. Bispecific antibodies in multiple myeloma treatment: a journey in progress. Front Oncol. 2022;12:1032775.
- Biopharma PEG. Bispecific antibodies - current status and prospects. www.biochempeg.com/article/252.html. Accessed February 10, 2023.
- American Journal of Managed Care. FDA approves bispecific antibody mosunetuzumab for R/R follicular lymphoma. www.ajmc.com/view/fda-approves-bispecific-antibody-monsunetuzumab-for-r-r-follicular-lymphoma. Accessed February 10, 2023.
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836-847.
- US Food & Drug Administration. FDA approves emicizumab-kxwh for prevention and reduction of bleeding in patients with hemophilia A with factor VIII inhibitors. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-emicizumab-kxwh-prevention-and-reduction-bleeding-patients-hemophilia-factor-viii#:~:text=On%20November%2016%2C%202017%2C%20the. Accessed February 10, 2023.
- US Food & Drug Administration. FDA approves emicizumab-kxwh for hemophilia A with or without factor VIII inhibitors. www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-emicizumab-kxwh-hemophilia-or-without-factor-viii-inhibitors. Accessed February 10, 2023.
- US Food & Drug Administration. FDA grants accelerated approval to amivantamab-vmjw for metastatic non-small cell lung cancer. Updated May 21, 2021. www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-amivantamab-vmjw-metastatic-non-small-cell-lung-cancer. Accessed February 10, 2023.
- US Food & Drug Administration. FDA approves teclistamab-cqyv for relapsed or refractory multiple myeloma. Updated October 25, 2022. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-teclistamab-cqyv-relapsed-or-refractory-multiple-myeloma. Accessed February 10, 2023P.
- Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385:1196-1206.
- American Journal of Managed Care. FDA approves faricimab to treat wet AMD and DME. www.ajmc.com/view/fda-approves-fariximab-to-treat-wet-amd-and-dme. Accessed February 10, 2023.
- Ben-Ari E. Teclistamab shows promise for people with heavily pretreated multiple myeloma. www.cancer.gov/news-events/cancer-currents-blog/2022/tecvayli-multiple-myeloma. Accessed February 10, 2023.
- Moreau P, Garfall AL, van de Donk N, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387:495-505.
- Searle E, Quach H, Wong SW, et al. Teclistamab in combination with subcutaneous daratumumab and lenalidomide in patients with multiple myeloma: results from one cohort of MajesTEC-2, a phase 1b, multicohort study. Presented at: ASH Annual Meeting, December 10-13, 2022; New Orleans, LA. Abstract 160.
- American Journal of Managed Care. From lymphomas to myeloma, bispecific antibodies make a big splash at ASH. www.ajmc.com/view/from-lymphomas-to-myeloma-bispecific-antibodies-make-a-big-splash-at-ash. Accessed February 10, 2023.
- Lesokhin AM, Arnulf B, Niesvizky R, et al. Initial safety results for MagnetisMM-3: a phase 2 trial of elranatamab, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients (pts) with relapsed/refractory (R/R) multiple myeloma (MM). Presented at: ASCO Annual Meeting, June 3-7, 2022; Chicago, IL. Abstract 8006.
- Bahlis NJ, Tomasson MH, Mohty M, et al. Efficacy and safety of elranatamab in patients with relapsed/refractory multiple myeloma naïve to B-cell maturation antigen (BCMA)-directed therapies: results from cohort a of the Magnetismm-3 study. Presented at: ASH Annual Meeting, December 10-13, 2022; New Orleans, LA. Abstract 159.
- Landgren O, Kazandjian D, O’Connell A, Finn G, Raje N. Magnetismm-4: an open label, phase 1B/2 umbrella study of elranatamab in combination with other anti-cancer treatments for patients with multiple myeloma. Presented at: ASH Annual Meeting, December 10-13, 2022; New Orleans, LA. Abstract 4567.
- Grosicki S, Mellqvist UH, Pruchniewski L, et al. Elranatamab in combination with daratumumab for patients (pts) with relapsed/refractory multiple myeloma (RRMM): results from the phase 3 Magnetismm-5 study safety lead-in cohort. Presented at: ASH Annual Meeting, December 10-13, 2022; New Orleans, LA. Abstract 1921.
- Hernandez GH, So J, Logronio KA, et al. Pharmacodynamics and biomarker correlates of imvotamab (IGM-2323), the first-in-class CD20xCD3 bispecific IgM antibody with dual mechanisms of action, in patients with advanced B cell malignancies. Presented at: ASH Annual Meeting, December 10-13, 2022; New Orleans, LA. Abstract 2865.
- Ferreri CJ, Quatela SE, Aina S, et al. Trial in progress: a phase II window of opportunity study of the BCMAxCD3 bispecific antibody REGN5458 in previously untreated patients with symptomatic multiple myeloma. Presented at: ASH Annual Meeting, December 10-13, 2022; New Orleans, LA. Abstract 4551.
- Otero PR, Joseph NS, Kumar SK, et al. Trial in progress: REGN5458, a BCMAxCD3 bispecific antibody, in a phase Ib multi-cohort study of combination regimens for patients with relapsed/refractory multiple myeloma. Presented at: ASH Annual Meeting, December 10-13, 2022; New Orleans, LA. Abstract 1936.
- Lesokhin AM, Richter J, Trudel S, et al. Enduring responses after 1-year, fixed-duration cevostamab therapy in patients with relapsed/refractory multiple myeloma: early experience from a phase I study. Presented at: ASH Annual Meeting, December 10-13, 2022; New Orleans, LA. Abstract 1924.
- Dekhtiarenko I, Lelios I, Attig J, et al. Intravenous and subcutaneous administration of RG6234, a novel GPRC5DxCD3 T-cell engaging bispecific antibody, is highly active in patients with relapsed/refractory multiple myeloma (RRMM): biomarker results from a phase I study. Presented at: ASH Annual Meeting, December 10-13, 2022; New Orleans, LA. Abstract 4554.
- Mazahreh F, Mazahreh L, Schinke C, et al. Risk of infections associated with the use of bispecific antibodies in multiple myeloma: a pooled analysis. Presented at: ASH Annual Meeting, December 10-13, 2022; New Orleans, LA. Abstract 1909.
- Catamero D, Martin T. A promising future for treating multiple myeloma with bispecific antibodies 2023. https://issuu.com/international-myeloma-foundation/docs/imf_06232022_slide_deck. Accessed February 20, 2023.
- Reed LM. Defining the role of the oncology nurse navigator. www.jons-online.com/issues/2020/march-2020-vol-11-no-3/2842-defining-the-role-of-the-oncology-nurse-navigator#:~:text=Oncology%20nurse%20navigators%20(ONNs)%2C,as%20education%20on%20treatment%20options%2C. Accessed February 20, 2023.
- Association of Community Caner Centers. Bispecific antibodies checklist for community providers. www.accc-cancer.org/docs/projects/bispecific-antibodies/checklist-for-bispecific-antibodies-jan-2022.pdf?sfvrsn=ad2f3ee4_2. Accessed February 20, 2023.
- Association of Community Caner Centers. Using bespecific antibodies in community practice: challenges and opportunities. www.accc-cancer.org/docs/projects/bispecific-antibodies/using-bispecific-antibodies-in-community-practice.pdf?sfvrsn=f5b5ac13_0. Accessed February 20, 2023.
- Atembina L, Boehmer L, Terrell K, et al. Multidisciplinary provider insights to promote adoption of bispecific antibodies to treat cancer in the community. Presented at: Association of Community Cancer Care, December 8, 2021. Abstract 4033.